Alexandra Duffy, D.O.
Brief Biography
 Dr. Duffy is a board certified neurologist with expertise in movement disorders and a particular interest in Huntington’s disease. After serving as Chief Resident in Neurology at UC Davis, she completed her Fellowship in Movement Disorders and now serves as an Assistant Clinical Professor of Neurology at UC Davis, where she also directs the Neurology Residency Program.
Dr. Duffy is a board certified neurologist with expertise in movement disorders and a particular interest in Huntington’s disease. After serving as Chief Resident in Neurology at UC Davis, she completed her Fellowship in Movement Disorders and now serves as an Assistant Clinical Professor of Neurology at UC Davis, where she also directs the Neurology Residency Program.
Dr. Duffy’s research has focused on Huntington’s disease. She served as a member of the clinical team for a research study called PRE-CELL, an observational study following a group of participants with early stage Huntington’s disease. She helped create a bioethics interview questionnaire for participants and their care partners aimed at understanding their expectations and experiences during the course of the study.
Along with her work in PRE-CELL, Dr. Duffy created a survey for persons at-risk for Huntington’s disease regarding their attitude, beliefs, and concerns about predictive genetic testing. She has presented this work at the Huntington Study Group Conference. She also conducted a survey for persons in the Huntington’s disease community to better understand their attitudes and concerns regarding novel treatments in Huntington’s disease so to inform the design of clinical trials for this population in future studies.
Research Overview
CIRM Grant DR2A-05415
In 2012, the California Institute for Regenerative Medicine awarded a disease-team grant to Dr. Vicki Wheelock and co-PI Dr. Jan Nolta entitled Mesenchymal Stem Cells Engineered to produce BDNF as a treatment for HD, with the goal of obtaining FDA approval and completing a Phase 1 safety and tolerability trial of MSC/BDNF in early-stage HD patients.
PRE-CELL is a prospective longitudinal observational lead-in study following a cohort of early-stage HD patients who are potential candidates for the planned cellular therapy trial. The primary objective is to establish the rate of change in clinical, imaging and biomarker measures in subjects. Subjects and their care partners were screened and then enrolled if all inclusion/exclusion criteria were met, and then were evaluated every 6 months over the next 12 to 30 months using a comprehensive battery of measures including neurological exams, cognitive and psychiatric assessments, MRI brain scans, and CSF/plasma analyses for levels of mutant huntingtin protein and BSNF
Preliminary Findings
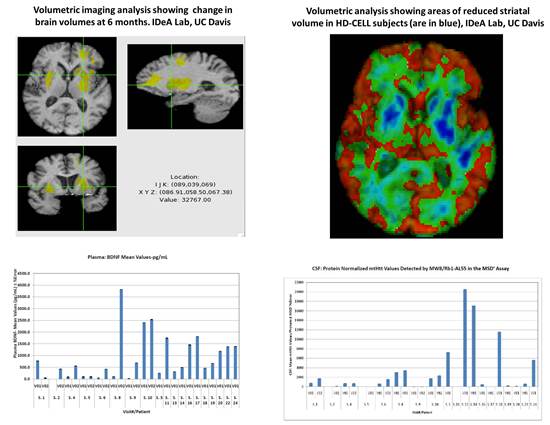
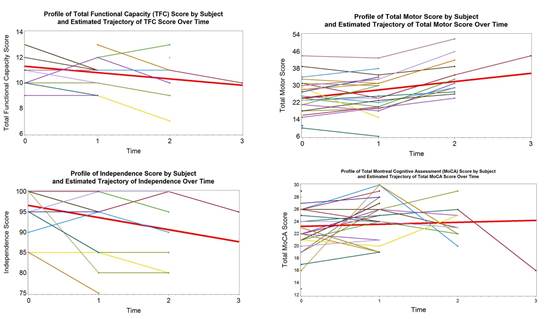
Future Directions
The PRE-CELL study closed at the end of July, 2016. We are currently completing final data analysis and preparing for publication. We are continuing protocol development for the planned Phase 1 open-label dose-escalation trial of MSC/BDNF, called HD-CELL. Ongoing studies in animal models are underway at the UC Davis Institute for Regenerative Cures to support our application for the Investigational New Drug trial. We hope to offer the opportunity to participate in the planned Phase 1 trial to PRE-CELL participants who completed at least one year of longitudinal assessments during the study.

