Vicki L. Wheelock, M.D.
Brief Biography
 Dr. Wheelock is a board-certified neurologist specializing in movement disorders. She is the director of the UC Davis Huntington's Disease Center of Excellence and a member of the Huntington Study Group.
Dr. Wheelock is a board-certified neurologist specializing in movement disorders. She is the director of the UC Davis Huntington's Disease Center of Excellence and a member of the Huntington Study Group.
After receiving her B.S. in Biomedical Sciences from the University of Michigan, she then earned her M.D., also at the University of Michigan. She completed her internship in Internal Medicine at William Beaumont Hospital, followed by her residency at the University of Southern California Medical Center.
She began the UC Davis Huntington's disease program in 1997, founding an interdisciplinary clinic recognized as an HDSA Center of Excellence since 2001. She has been an investigator for the Huntington Study Group since 1997, has served as a member of the HSG Executive Committee, and has served as a site investigator for many multi-center trials.
In 2009 she began collaborating with Dr. Jan Nolta, Director of the UC Davis Stem Cell Program, to seek potential therapies for Huntington's disease, and in 2012 they were awarded a CIRM grant to investigate Mesenchymal Stem Cells Engineered to Produce BDNF for the Treatment of Huntington's Disease.
Research Overview
Mesenchymal stem cells (MSCs) engineered to overexpress brain-derived neurotrophic factor (BDNF) is a proposed therapeutic for neuroprotection in Huntington’s disease (HD). PRE-CELL is the lead-in observational study for a future planned Phase 1 cellular therapy trial of MSC/BDNF in HD (NCT01937923). The primary objective was to enroll a cohort of up to 40 subjects with early-stage Huntington’s disease (HD) to characterize the rate of change in clinical, neuro-imaging, laboratory and biomarker correlates of disease progression over a 12 – 30 month period.
Subjects were recruited from July 2013 – June 2015 with the following inclusion criteria: adult men and women with clinically diagnosed HD, CAGn > 37, TFC 9 – 13, without dementia or unstable psychiatric symptoms, and with a care partner willing to enroll in the study and to report on their status. Exclusion criteria included pre-manifest or prodromal HD, minors, advanced HD clinical, stage, history of substance abuse, unstable psychiatric status, dementia, unstable general health, coagulopathy, history of previous stem cell or cytokine treatment, and contraindication to MRI.
Assessments were performed at screening/baseline and then every 6 months, including vital signs, clinical and neurological examinations, concomitant medication review, UHDRS motor examination, Total Functional Capacity, Functional Examination and Independence scores, cognitive assessment using the CAB-HD battery, Columbia Suicide Rating Score, Problem Behaviors Assessment-short form, HD Quality of Life, Neuropsychiatric Inventory-Caregiver, E-COG assessment, modified Rankin Scale, serum and CSF biomarkers, structural MRI and safety laboratories.
Statistical analysis was performed using a linear mixed effects model to analyze the repeated measures of the following selected clinical outcome measures in the cohort.
Research Program Details and Interim Findings
42 subjects were screened and 32 enrolled. Interim analysis of study measures for the subjects meeting enrollment criteria was performed in month 30 of the study, with 32/32 subjects completing 6 months of follow-up visits, 23 completing 12 months, 18 completing 18 months, and 7 completing 24 months.
The estimated change rates for selected clinical measures are shown below in Figure 1.
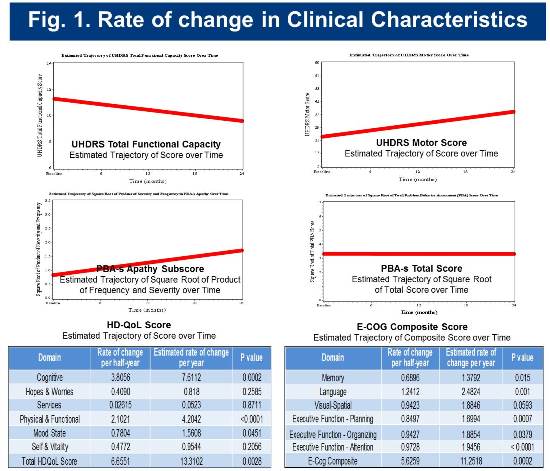
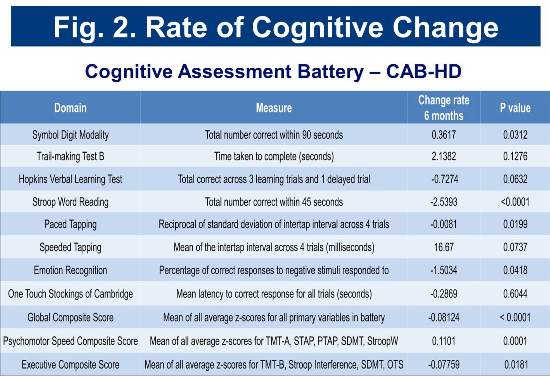
Baseline and longitudinal cognitive assessment scores for the cohort are consistent with those of cohorts identified as early-diagnosis in previous studies (Track-HD and CAB-Beta). Rates of change in selected cognitive measures are presented in Figure 2.
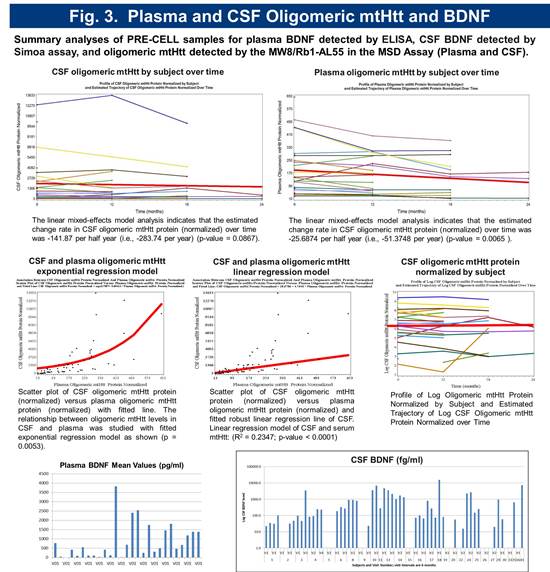
Preliminary biomarker analysis revealed significant variability in serum BDNF levels. CSF BDNF levels were below detection with standard assays. A novel ultra-sensitive Single Molecule Array (Simoa) based BDNF assay with a LLoQ of 50 femtogram/mL, which is > 300 fold more sensitive than current immunoassays was employed to quantitate CSF BDNF. Using this method, BDNF was detectable in 69% of subjects (Figure 5). Serum and CSF toxic oligomeric mutant Huntingtin protein (mtHtt) levels were measured using a novel Meso Scale Delivery (MSD) platform with antibodies MW8 and Rb1-AL55. The relationship between oligomeric mtHtt levels in CSF and serum was studied with fitted exponential regression model as shown (p = 0.0053) (Figure 3).
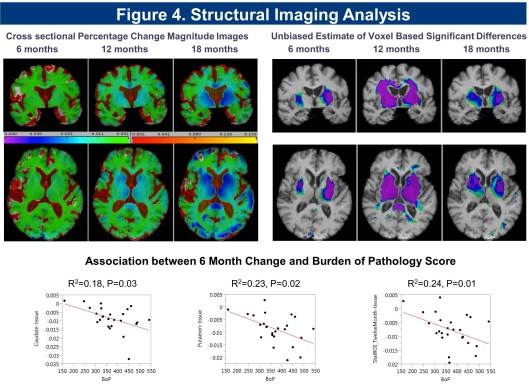
Cross sectional and longitudinal analysis of imaging data for the cohort shows significant reduction in striatal, putaminal region, and white matter volume, and increase in CSF volume detected at 6 months, with a significant correlation in rate of change seen with Burden of Pathology (BoP) scores (Figure 4).
Conclusions and Future Directions
The PRE-CELL study has successfully enrolled a cohort of subjects with early-stage HD and characterized the rate of change in clinical, imaging and biomarker measures. The estimated rate of change in TFC, independence score, functional check lists, PBA-s apathy subscore and CAB-HD battery are similar to findings seen in TRACK-HD and CAB-HD studies. The estimated rate of change in the HD-QOL, E-COG, and the modified Rankin score are also statistically significant at 6 months. We have detected novel findings in plasma and CSF biomarkers, including first measurements of CSF BDNF in HD subjects, as well as detection of oligomeric mtHTT. Cross sectional and longitudinal rates of change in structural imaging are robust at 6, 12 and 18 months and correlate with CAP scores.
The study has been closed. Final data analysis is nearly complete. These data will be used as a baseline for comparison in subjects who may enroll in the future planned Phase 1 trial once regulatory approval has been obtained and may be generalizable to other studies in early-stage HD.

