Study finds new treatment for pulmonary hypertension holds promise
Restoring growth suppressor may lead to reversing existing pulmonary arterial hypertension
A study led by The Goncharova lab at UC Davis Health has validated a potential therapy to treat pulmonary arterial hypertension. The condition is ultimately fatal and currently lacks effective treatments.
According to the study published in Science Signaling, restoring the functional growth suppressor tuberous sclerosis complex 2 (TSC2) may help to reverse existing pulmonary vascular remodeling and pulmonary arterial hypertension.
About 500-1,000 new cases of pulmonary arterial hypertension are diagnosed each year in the United States, according to the American Lung Association.
Pulmonary arterial hypertension is caused when the tiny arteries in the lung become thickened and narrowed. This restricts blood flow through the lungs, which raises the blood pressure in the lungs and causes the heart to work harder to pump blood. Over time, the heart loses the ability to effectively pump blood throughout the body.
“Pulmonary arterial hypertension is a rare and progressive disorder with a high mortality rate and no cure,” explained Elena A. Goncharova, professor of internal medicine and director of the Pulmonary Vascular Disease Program. “The condition is a serious public health problem with increasing death and hospitalization rates.”
Utilizing TSC2 for therapeutic intervention
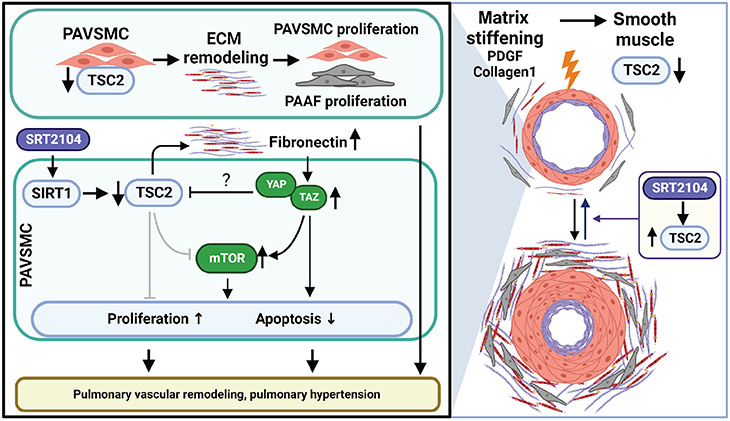
Pulmonary arterial hypertension is partially driven by the growth of pulmonary arterial vascular smooth muscle cells, induced by the stiffening of pulmonary arteries.
In the study, researchers aimed to see if they could find a way to open small pulmonary arteries to selectively kill modified smooth muscle cells and reverse pulmonary arterial hypertension.
The team discovered the growth suppressor TSC2 is virtually absent in vascular smooth muscle cells in small pulmonary arteries. Additionally, they found that TSC2 controls cell growth caused by mechanical biological cues — meaning if it is present, it can block the stiffening and remodeling of pulmonary arteries.
Ultimately, this discovery was more than we had hoped for. We realized by bringing back the growth suppressor TSC2, we could potentially return the arteries in the lung to a biological level.” —Elena A. Goncharova
“Ultimately, this discovery was more than we had hoped for,” Goncharova explained. “We realized by bringing back the growth suppressor TSC2, we could potentially return the arteries in the lung to a biological level.”
Next, the team began to evaluate potential medication strategies to restore functional TSC2 in pulmonary arterial vascular smooth muscle cells. Because TSC2 is regulated by the protein SIRT1, they tested the effect of the SIRT1 activator SRT2104, a drug that is currently in clinical trials as a therapeutic target in multiple age-related diseases.
“We found that treating pulmonary arterial vascular smooth muscle cells in rodents with SRT2104 significantly increased TSC2 protein levels, improving lung function and reducing pulmonary hypertension,” Goncharova said. “These results are very exciting and show that increasing TSC2 abundance could offer a new target for therapeutic intervention for a condition that is ultimately fatal and currently lacks effective treatments.”
Support and funding
The researchers are grateful to the Small Animal Hemodynamic Core at the University of Pittsburgh Heart, Lung, and Blood Vascular Medicine Institute for the acquisition and analysis of animal hemodynamic data. Lung tissues and pulmonary vascular cells from patients with pulmonary arterial hypertension and unused donor lungs were provided by the Pulmonary Hypertension Breakthrough Initiative and the University of Pittsburgh VMI Cell Processing Core.
This work is supported by National Institutes of Health (NIH) and National Heart, Lung, and Blood Institute (NHLBI) grants R01HL113178, R01HL130261, R01HL150638, 5P01HL103455-05, 1 U01HL145550, 5 P50AR060780 and American Heart Association Postdoctoral Fellowship 826806.