UC Davis partners with Caring Cross and CIRM to develop immunotherapy for cancer patients
A Q&A with immunologist William Murphy on developing and testing new cancer CAR T-cell treatments
In December, William Murphy was thrilled to learn that his application to California Institute for Regenerative Medicine (CIRM) was approved for a $4 million grant. Now, he and a team of UC Davis researchers can develop and test a chimeric antigen receptor (CAR) T-cell therapy to treat various B-cell malignancies, ranging from lymphomas to leukemias.
Murphy is a distinguished professor and vice chair of dermatology. He also has a joint appointment with the Department of Internal Medicine, Division of Malignant Hematology/Cellular Therapy and Transplantation.
In this Q&A, he discusses the importance of T-cell therapy and its implications for developing cancer treatments. His work is a collaboration between the nonprofit organization Caring Cross, CIRM and UC Davis Health.
What are B-cell malignancies?
B-cells are a type of white blood cells that make antibodies. They are key to the body’s immune system. When healthy B-cells change into fast-growing cancer cells that don't die, they cause B-cell malignancies.
This can affect people at different ages. They may show up in children as B-cell acute lymphoblastic leukemia (B-ALL), an aggressive blood and bone marrow cancer. In adults, they make up about 85% of non-Hodgkin lymphoma (NHL), a cancer that starts in B lymphocytes. In the elderly, B-cell malignancies may come as multiple myeloma, a cancer of the plasma cells.
There are different lines of treatments for B-cell lymphoma and leukemia, including immunotherapy using chimeric antigen receptor (CAR) T cells. These cells have revolutionized cancer treatment since they have been shown to work, and cure, when nothing else can.
What is chimeric antigen receptor (CAR) T-cell therapy?
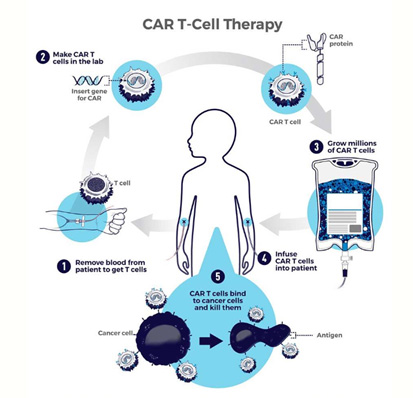
Chimeric antigen receptor (CAR) T-cell therapy uses the body’s own defenses to fight disease. It is a new and exciting form of immunotherapy that works by modifying the receptors of immune cells (T cells) involving antibodies to target specific cancers, such as leukemias and lymphomas.
CAR T cells are being used to treat some blood cancers with long-term success. The U.S. Food and Drug Administration (FDA) first approved CAR T-cell therapy in 2017. Their use is growing rapidly and being applied to other tumor types. Yet, this therapy is extremely expensive, even with insurance. It's also a very intensive procedure and it takes time to generate the CAR T cells from the patient.
While it could be considered a game changer, one of the issues with this therapy is the case relapse rate. The big holy grail in cancer therapy is how to prevent tumors from evading or escaping the immune attack. Around 60% of patients who get CAR therapy see their cancer return. If we can get the relapse rate down to negligible, that would be a tremendous advance.
How do you intend to use CAR products to reduce cancer relapse?
In CAR therapy, we take the immune T cells from a patient and use gene therapy to give a new receptor to signal and direct the T cell. The receptor usually has an antibody that recognizes a particular tumor antigen. Current FDA-approved CAR T therapies only target one tumor antigen.
CARs have had tremendous success. However, there is significant patient relapse because the tumor adapts and may lose that one antigen that we are targeting, allowing it to escape the treatment. Our strategy is to target multiple antigens to reduce the potential for relapse since the tumor cannot adapt that quickly.
Around 60% of patients who get CAR therapy see their cancer return. If we can get the relapse rate down to negligible, that would be a tremendous advance."—William Murphy
We are also proposing a novel vector that will carry a CAR product, known as DuoCAR, that targets three antigens at the same time. As long as the tumor has one of the three antigens, then there's little chance for the tumor to escape all three antibodies. This is similar to when you think about HIV treatment with the triple-drug therapy, where one alone is not sufficient.
The hope is that the 60 to 70% of the population who would have relapsed if they had the original CAR T cell treatment, would have a home run with our kind of treatment or product.
So, is this treatment for cancer patients who have relapsed?
We see this product as a new frontline therapy and not just for patients who relapse. What the patient has to go through in order for CAR T therapy to work is very strenuous. So, yes, if there are relapsed patients, they can be given DuoCAR, but we're also hoping this will become the new standard of care, replacing the other CARs in the future for everyone.

Who is manufacturing the Duo CAR?
Joseph Tuscano, interim director of the UC Davis Stem Cell and Bone Marrow Transplantation Program, approached me about taking this CAR T approach to the next level by doing immediate onsite CAR T cell production. We at UC Davis will be the first in the country to do this process with this specific CAR in patients. We would generate the CARs ourselves - rather than have them sent to us. Doing everything in-house will markedly reduce the cost and increase efficiency. It will also save time for the patient.

To do that, we work closely with Jan Nolta at the Institute of Regenerative Cures, Mehrdad Abedi at the UC Davis Alpha Stem Cell Clinic and our renowned Good Manufacturing Practice (GMP) facility. They all have the expertise, resources and infrastructure.
Most clinical applications involve obtaining CARs from other sources. This means the cells come frozen. Most people agree fresh is better, as freezing can damage the cell. The advantage of doing everything in-house is you don't have to freeze the cells, and it saves time.
However, you always want to keep some cells frozen for later - just in case the tumor does come back. The question is, can we reapply in the same patient? That's something that we're starting to look at that others really haven't done before. You can have a reserve that you can then draw back on, even if it's five or 10 years later. Also, studying CAR T cells’ lifespan is something that the FDA wants to see. So, we will be doing preclinical studies to understand what happens with CARs over time and how to make them even better.
It puts California at the forefront of this cutting-edge therapy. Through this collaboration, we're hoping to set the stage for newer generation vectors and CAR T cells for other kinds of cancers and even outside of cancer, such as viral infections or autoimmunity."—William Murphy
Who are you working with on this project?
This work is a collaboration between Caring Cross, a scientific foundation, and UC Davis and is funded by CIRM.
Caring Cross’ mission is to make cancer treatments like CAR T cell therapies accessible for all. They have a team of scientists but lack the resources to do the testing, especially for the FDA and clinical trials. This requires an academic medical center like ours at UC Davis Health.
UC Davis wants to be at the cutting edge of medical innovation and provide our patients with resources no one else has. We especially want to make this available to our entire catchment area. We are uniquely positioned to serve and engage with diverse, underserved and marginalized communities.
CIRM wants to make stem cells or gene therapy treatments available for California citizens. So, everything aligns nicely.
Combining these three resources allows sufficient resources and time to be put into what no one place could really do on its own. It puts California at the forefront of this cutting-edge therapy. Through this collaboration, we're hoping to set the stage for newer generation vectors and CAR T cells for other kinds of cancers and even outside of cancer, such as viral infections or autoimmunity.
How does CIRM’s grant help you take your next steps?
CIRM’s grant is significant for cancer patients, our university, and California.
It allows us to be ready for the next stage – the submission to the Pre-Investigational New Drug Application (Pre-IND) to the FDA. With the funding, we will address the safety and production issues before attempting clinical translation.
This grant will also help establish UC Davis as a one-stop shop. The goal is to help generate the data for preclinical, go through the FDA for clinical approval, do the trials here and then expand it.
As for the steps, this study is expected to go very fast. The first step is testing the vector to see if it can carry the DuoCAR. Although we know the vector works, the FDA wants us to study the product under all kinds of conditions and conduct toxicity studies.
Based on what we and our collaborators have seen, this product works great, but that's in mouse models. Now, we have to show that the product is safe, prevents relapse, and, when scaled up, still works well.
Depending on how everything goes, we're looking at getting DuoCAR into patients in two years, maybe less. Our work is bench to bedside, back to bench. This way, we continue to improve it, learning from what happens in the patient.
How do you see the field of CAR T- cell therapy evolving?
There is an explosion in the field in how we make CAR technology better. You almost have to marry up different disciplines: the clinical oncologist, the immunologist, the cancer biologist, and then the molecular biologist to really get the optimal product. What’s also interesting about CAR T cells and CAR technology is that the clinical trials are almost outpacing the basic science.

CAR therapies are not just being used for cancer. A CAR clinical trial for HIV is underway at UC Davis, and groups are looking at CAR therapies for autoimmunity and other disorders. My laboratory’s role, as immunologists, is to understand how to make the CAR T cell itself better in the body.
Also, we're seeing a change from only big cancer centers developing and giving CAR therapy. Now, any comprehensive cancer center is expected to be able to give this therapy as a resource and develop it - not just apply it. The technology is becoming more and more accessible, especially with these kinds of collaborations. That only helps the patients because that means the physicians will be more comfortable applying, using and developing it.
There is no going back in CARs. Soon, it will be a matter of whether we can apply it to more kinds of cancer and make it more effective and less costly.
Related Stories: