The patient was an otherwise healthy woman in her 40s, who transferred to UC Davis Medical Center after a community hospital was unable to slow her respiratory infection. Within 24 hours, her status deteriorated more.
Chest imaging suggested community-acquired pneumonia, but UC Davis clinicians immediately suspected something else — a potential COVID-19 infection.
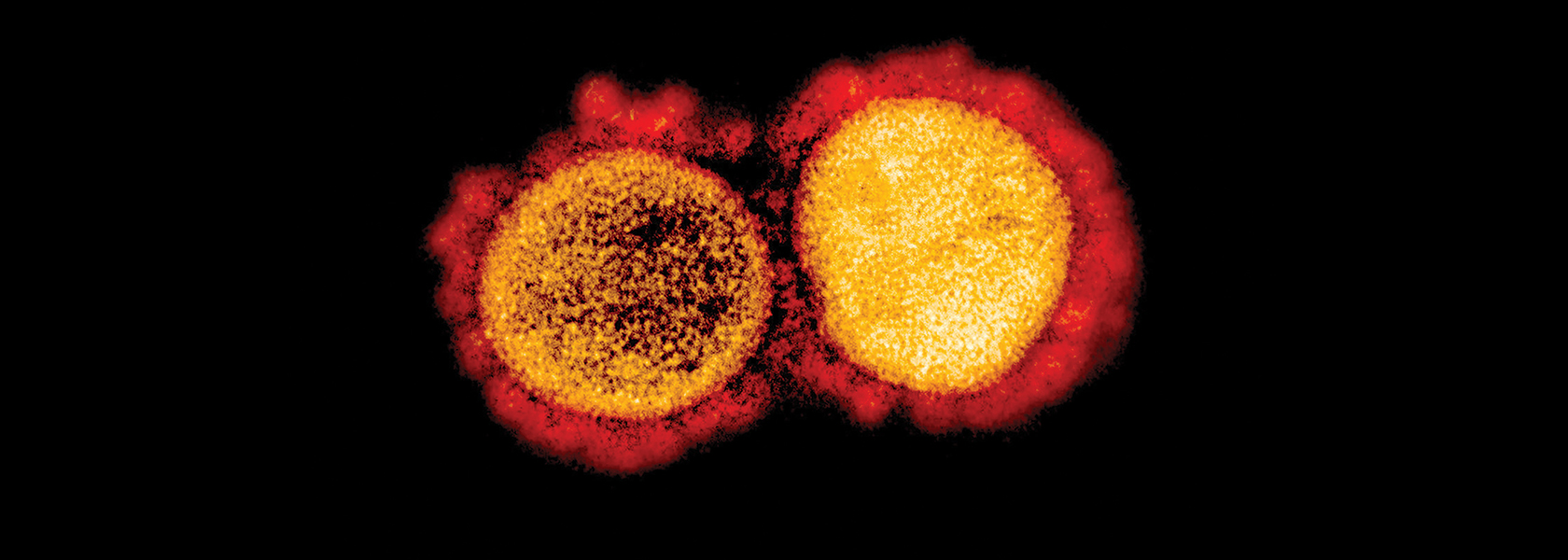
At that time — early-to-mid February — the novel coronavirus was picking up steam, fast. In January, the World Health Organization had declared a global health emergency after thousands of cases and more than 1,000 deaths in China. And as cruise ships locked down and outbreaks emerged in Europe and the Middle East, UC Davis infection prevention specialists in Sacramento began surveilling their own patients for possible cases.
But at that point the virus was still considered largely an international affair by several governments, with travel warnings or restrictions a cornerstone of their infection control. And because UC Davis Health’s patient had not traveled to China or other high-risk countries, or had known contact with high-risk individuals, she didn’t meet criteria for testing by the U.S. Centers for Disease Control and Prevention.
Over the next several days, UC Davis clinicians proceeded to eliminate other potential causes of her acute respiratory distress syndrome and septic shock. But the viral panel and respiratory, blood and bronchoscopy cultures still failed to indicate a clear infectious source, too. So with all other options ruled out, the CDC authorized a COVID-19 test.
It came back positive two days later. The case would now be the nation’s first apparent example of community spread, and one of its first COVID-19 hospitalizations. And when UC Davis announced it on Feb. 26, it made headlines around the world (including in a CDC statement, which noted “This case was detected through the U.S. public health system — picked up by astute clinicians”).
UC Davis Health and UC Davis have remained in the thick of local, national and even global pandemic responses ever since, long after discharging that index patient. UC Davis teams are using their collective experience in health, virology, engineering, and zoonotic diseases to tackle the crisis at all levels — administering clinical care and trials; defining paths for transmission; bridging gaps in testing and equipment; providing safety guidance to the public; highlighting the importance of socioeconomic disparities; and more.
“Since that first day, every person at UC Davis Health has played a vital role as we confronted this unprecedented crisis,” said David Lubarsky, M.D., M.B.A., UC Davis Health’s CEO and the Vice Chancellor of Human Health Sciences for UC Davis. “They’ve shown the utmost grace under pressure, while serving our patients and collaborating with our community partners in the interest of public health — for everyone.”
Highlights: Responding to the novel coronavirus
December 2019
An outbreak of acute respiratory illness, linked to a novel coronavirus, emerges in China
January 2020
Jan. 21 – The U.S. confirms its first travel related COVID-19 case
Jan. 30 – The World Health Organization (WHO) declares a global health emergency
February

Feb. 26 – UC Davis treats the first U.S. community spread case
UC Davis Health announces it is treating the first apparent case of COVID-19 acquired by community spread, making headlines around the world and driving changes to national testing guidelines. UC Davis clinicians had initially suspected their transferred patient’s mystery illness was COVID-19, but were unable to confirm immediately due to the strict federal testing criteria of the time. In its wake, the CDC allows any hospitalized patient with severe symptoms to be tested, and allows national testing capabilities to expand to state and local health labs.
Feb. 29 – The first suspected U.S. death is reported near Seattle
March
Mar. 11 – The WHO declares a global pandemic
Mar. 13 – The U.S. declares a national emergency
Mar. 16 – California issues a sweeping stay-at-home order
Mar. 23 – Sacramento County issues a stay-at-home order
Mar. 26 – The U.S. takes over the world lead in reported cases
Mar. 30 – Sharing lessons from our index case
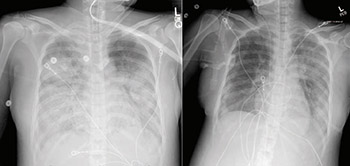
UC Davis Health clinicians publish a case study about their index patient in the journal Clinical Infectious Diseases. Multiple journal papers published about the index patient’s successful treatment help to educate providers — and save lives — at hospitals across the U.S. UC Davis hematopathologists reported leukemia-like blood test results from the case, for example, and advised fellow clinicians to order COVID-19 testing instead of cancer screenings for certain symptom sets.
Setting state and national COVID-19 standards
UC Davis Health faculty help to set comprehensive standards for pandemic-related patient care and workforce safety. Examples:
- Cardiothoracic surgeon David Cooke, M.D., F.A.C.S., assists in writing national COVID-19 guidelines for triage of thoracic surgery patients, through the American College of Surgeons (ACS) and thoracic societies.
- Surgery chair and ACS regent Diana Farmer, M.D., helps develop COVID-19 best practices and safety standards for surgeons.
- Otolaryngology chair Gregory Farwell, M.D., F.A.C.S., helps establish an American Head and Neck Society best-practices repository.
- Burn surgeons David Greenhalgh, M.D., and Tina Palmieri, M.D., and nurse manager Len Sterling, R.N., M.B.A., N.E.A.- B.C., help U.S. burn centers plan for continuous burn care during ICU surges.
- Pediatrics chair Satyan Lakshminrusimha, M.D., neonatology chair Mark Underwood, M.D., M.A.S., and pediatric infectious disease professors Elizabeth Partridge, M.D., M.P.H., and Jean Wiedeman, M.D., Ph.D., help co-author COVID-19 guidelines in the American Journal of Perinatology.
April
Apr. 6 – Boosting California’s testing capacity
After steadily increasing its own testing, UC Davis Health is named to California Gov. Gavin Newsom’s COVID-19 Testing Task Force, a public-private collaboration to more quickly increase statewide capacity. The health system can already run up to 400 tests per day, and is working to expand into a high-volume hub with its Roche Diagnostics cobas 6800 robot.
Apr. 16 – Reporting the first trial of a promising antiviral
Preliminary results emerge from the first small clinical trial of the antiviral therapy remdesivir, conducted at UC Davis Health and hospitals worldwide. Nearly two thirds of the severely ill, hospitalized COVID-19 patients who received the drug on a compassionate-use basis improved, with no new safety concerns. WebMD’s Chief Medical Officer interviews site principal investigator Stuart Cohen, M.D., about the results, published in the New England Journal of Medicine. Larger, controlled trials follow, and remdesivir emerges as one of the most promising in the early field of potential treatments.
Apr. 21 – A Santa Clara County resident who died in early February becomes the nation’s new earliest known victim
Apr. 28 – U.S. coronavirus deaths exceed those from the Vietnam War

Apr. 29 – Testing begins on a potential vaccine
Biopharmaceutical company Verndari, Inc. announces the start of preclinical testing at UC Davis’ Mouse Biology Program to evaluate a potential vaccine and dermal patch delivery system for COVID-19. The Napa-based company had joined the university’s START support program for UC Davis-affiliated entrepreneurs and startups in 2016.
The testing collaboration “illustrates one of many ways that UC Davis is leveraging our unique expertise and established platform, built on previous research for HIV, Zika and human cytomegalovirus, to advance knowledge and solutions specific to COVID-19,” says Prasant Mohapatra, Ph.D., the university’s vice chancellor for research.
Apr. 29 – Plasma transfusions for COVID-19
Two UC Davis Health patients with COVID-19 receive transfusions from a blood donor who recovered from the virus, as part of a national initiative investigating the potential benefits of convalescent plasma. It’s hoped the treatment may boost a sick patient’s ability to neutralize COVID-19 and its effects.
May
May 14 – Panel examines pandemic disparities
The UC Davis Health Office for Health Equity, Diversity and Inclusion hosts a panel to explain reasons for health disparities related to COVID-19, and what can be done about them. Physicians at “COVID-19: Addressing Health Disparities in the African American Community” say that social determinants of health are a major reason African Americans and Latinos in California are infected with COVID-19 at rates higher than whites. African Americans are dying at rates disproportionate to their population.
May 19 – Tracking a troubling syndrome in children
UC Davis Children’s Hospital becomes part of an international group researching multisystem inflammatory syndrome in children (MIS-C), a new form of COVID-19 likened to toxic shock syndrome and Kawasaki disease. UC Davis Health faculty helped establish a pediatric research group (PECARN) in the U.S., and serve as principal investigators for the international Pediatric Emergency Research Networks (PERN).
May 21 – 10,000th molecular test
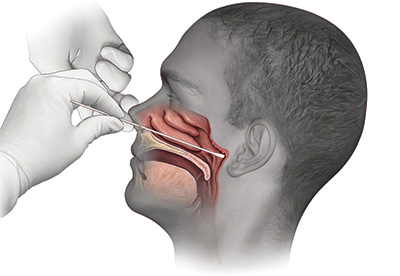 An illustration of the swab test distributed by UC Davis Health trended nationally in the early pandemic, ranking high on search engines.
An illustration of the swab test distributed by UC Davis Health trended nationally in the early pandemic, ranking high on search engines.Clinical pathologists perform their 10,000th test of COVID specimens from UC Davis patients and staff, as well as residents at nursing homes, local community hospitals and other long-term facilities that lacked testing capabilities. UC Davis is able to test all hospitalized patients at UC Davis Medical Center to help ensure safety at the regional tertiary and quaternary care facility. The health system also processes tests gathered at community sites — activity which helps identify that working-age Latinos are getting infected and dying in disproportionately high numbers.
May 26 – Highlighting policy challenges — and opportunities
In the Journal of Gerontological Nursing, nursing professor and aging expert Heather M. Young, Ph.D., R.N., F.A.A.N., summarizes a series of temporary, pandemic-related public policy changes that improve care access for older adults — and urges their permanent consideration. In an earlier editorial, she described in detail how COVID-19 underscores the unique needs of older adults and caregivers.
May 28 – U.S. COVID-19 deaths exceed 100,000
Early June
Post-Memorial Day surge begins
June 18–20
WHO and NIH halt trials of hydroxychloroquine
July
July 7 – U.S. withdraws from WHO
July 20 – Testing a monoclonal antibody cocktail
 UC Davis Health announces a federal grant to help test a new antibody combination as a possible therapy for reducing viral shedding and disease progression. The clinical trial seeks to evaluate efficacy and safety of REGN-COV2 — a combination of REGN10933+REGN10987 antibodies — in hospitalized adult COVID-19 patients. Trial sponsor Regeneron Pharmaceuticals developed the combination to bind to the SARS-CoV-2 spike protein and block its interaction with the host receptor. The adaptive phase I, II and III study is randomized, double-blinded and placebo-controlled.
UC Davis Health announces a federal grant to help test a new antibody combination as a possible therapy for reducing viral shedding and disease progression. The clinical trial seeks to evaluate efficacy and safety of REGN-COV2 — a combination of REGN10933+REGN10987 antibodies — in hospitalized adult COVID-19 patients. Trial sponsor Regeneron Pharmaceuticals developed the combination to bind to the SARS-CoV-2 spike protein and block its interaction with the host receptor. The adaptive phase I, II and III study is randomized, double-blinded and placebo-controlled.
July 23 – A new ICU partnership in a hotspot county
UC Davis Health and Adventist Health Lodi Memorial partner to launch a 24/7 telehealth link between the Lodi hospital’s intensive care unit — which has been caring for many COVID-19 patients in highly-impacted San Joaquin County — and university pulmonology and critical care physicians.
July 30 – Clinical guidance for providers everywhere
A primer on coronavirus and diagnostic error becomes the latest COVID-19 clinical guide released on the widely known safety clearinghouse website AHRQ Patient Safety Network (PSNet), co-edited since fall 2019 by internal medicine professor Patrick Romano, M.D., M.P.H., and nursing professor Debra Bakerjian, Ph.D., A.P.R.N., F.A.A.N., F.A.A.N.P., F.G.S.A. The two safety experts use their expertise to curate rapidly emerging information for busy clinicians, health academics and other stakeholders.
August
Aug. 12 – Part of a major vaccine trial
UC Davis Health announces a partnership with Pfizer Inc. and Germany based BioNTech SE to participate in a global study of an investigational vaccine against COVID-19, with the first of the university’s 200 vaccine candidate participants receiving their shots a week later.
The phase II/III trial, which involves 120 sites and roughly 30,000 people worldwide, seeks to determine the efficacy and side effects of a single nucleoside-modified messenger RNA candidate from the pharmaceutical companies’ BNT162 mRNA-based vaccine program.
The novel modified mRNA includes a piece of the genetic code of SARS-CoV-2, and is cited as the first time mRNA-based vaccines are used against an infectious disease.
The trial is designed as a 1:1 investigational vaccine candidate to placebo, randomized, observer-blinded study to obtain safety, immune response, and efficacy data for regulatory review. UC Davis Health site principal investigators include internal medicine chair Timothy Albertson, M.D., M.P.H., Ph.D., and Angela Haczku, M.D., Ph.D., associate dean for translational research.
Aug. 28 – Contact tracing for community health workers
UC Davis Health and two other universities receive a $2.3 million NIH grant to train and empower community health workers in research best practices such as contact tracing, which could help reduce disparities related to the pandemic.
September
Sept. 10 – Telehealth for at-risk Valley residents
Nursing and medicine professor Katherine Kim, Ph.D., M.P.H., M.B.A., co-leads the newly launched ACTIVATE initiative, a public-private pilot to bring telehealth services to underserved rural residents — such as pandemic-hit ag workers — in Merced County. The project, run through UC’s Center for Information Technology Research in the Interest of Society & the Banatao Institute (CITRIS), will also develop a roadmap for post-pandemic sustainability of health system reforms that incorporate telehealth.
As of late Sept.
(press time)
200,000+ U.S. coronavirus deaths
215,000 U.S. Civil War battle deaths*
291,557 U.S. WW2 battle deaths*
675,000 U.S. 1918 influenza deaths
*VA data
Keeping the public informed
 Throughout the pandemic, experts like pediatric infectious diseases chief Dean Blumberg, M.D., and public health sciences chair Brad Pollock, M.P.H., Ph.D., share context about the latest numbers, research, and prevention measures through news and web stories. Mental health professionals like Peter Yellowlees, M.B.B.S., M.D., and psychiatry chair Helen Kales, M.D., perform similar roles for related anxiety and depression.
Throughout the pandemic, experts like pediatric infectious diseases chief Dean Blumberg, M.D., and public health sciences chair Brad Pollock, M.P.H., Ph.D., share context about the latest numbers, research, and prevention measures through news and web stories. Mental health professionals like Peter Yellowlees, M.B.B.S., M.D., and psychiatry chair Helen Kales, M.D., perform similar roles for related anxiety and depression.
In the roughly six-month period after announcing the nation’s first community-spread COVID-19 patient, UC Davis Health registers more than 4 million views to its pandemic-related web content, and appears in more than 6,000 coronavirus-related media mentions.