New potential treatment for graft-versus-host-disease and other inflammatory disorders
Dual cytokine blockers cut toxic reactions after allogeneic stem cell transplantation
A new study led by UC Davis Health researchers showed that blocking IL-6 and TNF cytokines provides a more effective approach to preventing life-threatening graft-versus-host-disease (GVHD), an inflammatory condition that develops in patients after their allogeneic hematopoietic stem cell transplantation (allo-HSCT). The study was published today in Blood.
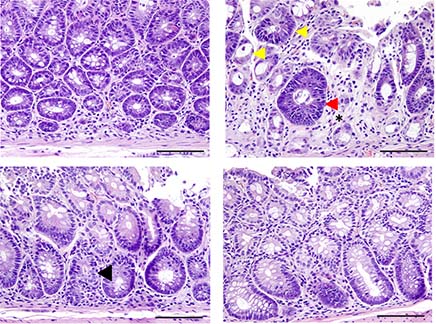
Allo-HSCT, a treatment for some blood disorders and cancers, entails infusing a donor’s bone marrow stem cells into patients undergoing intensive chemo and radiation therapy. The donated immune cells in the transplanted tissue (graft) initiate an immune response to the patient's tumor cells, leading to favorable graft-versus-tumor (GVT) effects. However, these cells may also attack the patient’s healthy tissues, resulting in potentially lethal graft-versus-host-disease.
What is graft-versus-host-disease?
GVHD is a significant cause of morbidity among allo-HSCT patients. It occurs when donor immune cells in the graft attack multiple organs and induce a “cytokine storm”, an inflated proinflammatory reaction caused by cytokines. Acute GVHD develops suddenly and shortly after transplantation. Chronic GVHD shows up later with delayed inflammation and may lead to tissue fibrosis.
“GVHD remains a major barrier limiting the success of the stem cell transplantation for cancer patients,” said Vice Chair of Research and Distinguished Professor at the Departments of Dermatology and Internal Medicine William Murphy, the senior author on the study.
From single to dual cytokine blockade
TNF and IL-6 are two cytokines that play a key role in many health conditions, including autoimmunity and excessive immune reactions to viruses, such as SARS-CoV-2, the virus causing COVID-19. Current therapies focus on blocking one of these cytokines to control the cytokine storm.
Targeting a single cytokine has been successfully used in certain cases, such as blocking TNF for autoimmunity conditions and IL6 for cytokine release syndrome after CAR T-cell immunotherapy and COVID-19 infections. However, simultaneously blocking both these cytokines has not been evaluated - until now.
“Surprisingly, only a single cytokine blockade to IL6 or TNF was evaluated in preventing GVHD, which gave disappointing clinical results. Given the important and possibly redundant roles these powerful inflammatory cytokines play, it made sense that combination approaches would be needed for greater efficacy,” Murphy said.
Our study demonstrated - for the first time - superior protection from GVHD when both TNF and IL-6 blockade were combined” —William Murphy
New treatment approach for better protection
The current study tested the combined TNF and IL-6 treatment on obese mouse models after allo-HSCT where the cytokine release syndrome is severe. Recent research from Murphy and his team showed that host conditions, such as obesity, can worsen acute GVHD due to a further increased cytokine storm. The role obesity plays in cytokine storm flares was also observed in COVID-19 cases and other conditions.
The study found that blocking both cytokines led to superior protection against acute GVHD severity and mortality, particularly affecting the gastrointestinal tract, one of the major target organs in GVHD. Importantly, the combined treatment did not impair graft-versus-tumor (GVT) effects.
“Many treatments for GVHD involve suppressing the body’s immunity, which can inhibit beneficial GVT effects. For this reason, it was important to determine if blocking these cytokines impacted the GVT response. Fortunately, anti-tumor effects remained after the transplant and with the combined intervention,” said Lam T. Khuat, first author on this study and a postdoctoral researcher at UC Davis Health.
This study may help in finding better treatments for proinflammatory diseases such as COVID-19. Currently, only IL-6 blockers are used to prevent the cytokine storm caused by the body's excessive immune reaction to the coronavirus.
“The increased efficacy of combined blockade suggests that this approach to treating diseases caused by inflammation may have greater benefit in other clinical settings,” Murphy said.
UC Davis co-authors are Logan V. Vick, Cordelia Dunai, Craig P. Collins, Catherine T. Le, Chien-Chun Steven Pai, Kevin M. Stoffel, Emanual Maverakis, Shyam K. More, Maneesh Dave, Robert J. Canter, Arta M. Monjazeb, Mehrdad Abedi, and Eunju Choi. Other co-authors are Dan L. Longo at the Harvard Medical School and Bruce R. Blazar at the University of Minnesota.
Funding: This work was funded by National Institutes of Health grants (R01 CA214048, R37 AI34495, HL56067) and the UC Davis Comprehensive Cancer Center Support Grant (CCSG) (P30CA093373).