How understanding the secrets of herpesviruses helps fight cancer
At UC Davis Comprehensive Cancer Center, one scientist’s sleepless nights are driven by the mysteries of virus-host interactions. Researcher Yoshihiro Izumiya is delving into the complex world of herpesviruses. These viruses often lie dormant for years, then wake up to wreak havoc on their hosts, causing severe diseases and complications.
Herpesviruses are common pathogens with more than 100 known types. Eight primarily affect humans. These human herpesviruses (HHVs) include the sexually transmitted herpes simplex viruses (types 1 and 2), Epstein-Barr virus and Kaposi’s sarcoma-associated herpesvirus (KSHV).
While many people carry these viruses without knowing it, the infections can remain hidden for years before suddenly causing serious health issues.
“Causing disease to the host is not a smart thing for pathogens. They draw attention to their presence and invoke a response from the host’s immune system. The smartest viruses just infect and don’t induce host immune reactions,” Izumiya said.
Izumiya is a professor in the Department of Dermatology and serves as a staff research scientist at the cancer center. His lab studies virus-host interactions, focusing on how herpesviruses shift from dormant to active replication, often resulting in diseases.
The smartest viruses just infect and don’t induce host immune reactions.” —Yoshihiro Izumiya
Switching from dormant to active state of replication
One line of research at Izumiya Lab relates to the Kaposi’s sarcoma herpesvirus. This virus is known for its link to Kaposi’s sarcoma, a skin cancer, and AIDS-related Castleman disease, a rare disease involving enlarged lymph nodes.
Izumiya’s team is working to understand how KSHV's chromatin, a genetic material, remains in a ready-to-reactivate state and how it becomes active with external stimuli. By studying these mechanisms, they hope to develop treatments that could prevent the virus from reactivating.
“The key to identify a therapeutic approach for KSHV-associated diseases lies in understanding when and how KSHV starts to replicate from dormant viral chromatins,” Izumiya explained. “We’re trying to uncover the mechanisms behind this shift and focus on how the host inflammation supports KSHV replication and disease development cycles.”
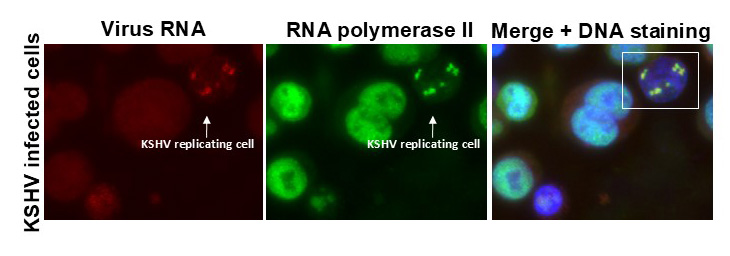
Herpesvirus hijacking the cell’s machinery
Herpesvirus is a remarkably complex virus. It can’t replicate on its own outside of infected host cells. Therefore, it mastered to command multiple cell’s machinery.
Izumiya and his team are investigating how KSHV manipulates the host cell’s functions. The researchers have found specific viral proteins essential for hijacking the cell resources.
In cells infected with KSHV, the virus hijacks the cell’s transcriptional machinery, which generates messenger RNAs (mRNAs). KSHV replication turns off the host cell mRNA expression by redirecting the cell resources to produce viral mRNAs. This mechanism prioritizes virus replication.
The team has also taken a “reverse hijacking” strategy and identified a small protein domain, called VGN50. VGN50 controls the cell’s transcription processes like a viral protein does.
“When the virus is replicating, the cell stops growing. The virus practically takes over the transcriptional apparatuses,” Izumiya explained. “By borrowing the virus’s hijacking mechanism with VGN50, we can slow cell inflammatory responses.”
With a $240,000 grant from the American Cancer Society, the team will further develop VGN50 as a potential drug.
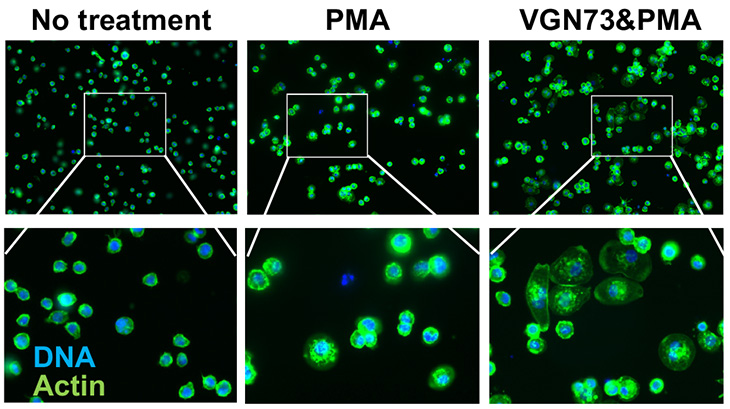
Recently, Izumiya Lab has prepared another special peptide named VGN73. VGN73 also borrows from the viral protein's mechanism to limit mRNA production. Their study was published today in Cell Chemical Biology.
These research projects on viral-host interaction have great implications for cancer studies. The team will test the small peptide identified from KSHV protein sequence to halt the cancer cell growth. Studies on viral-host interactions are important to find new drugs, Izumiya said.
Herpesvirus linked to neurological diseases
Izumiya’s work is not limited to KSHV. His team is also investigating herpesviruses 6A and 6B, collectively known as HHV-6A/B. These viruses infect people from a very young age. The viral genome integrates in germ cells and can pass from one generation to the next.
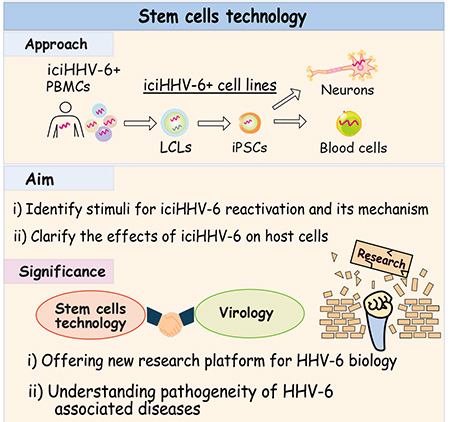
“This inherited form of HHV-6A/B is found in around 1-2% of the population in Europe and the U.S. The entire virus genome becomes part of every cell chromosome,” Izumiya said. “Clinical observation suggests that inherited chromosomally integrated HHV-6 (iciHHV-6) is linked to a range of serious health issues. Those include brain inflammation, pre-eclampsia in pregnancy and complications following stem cell transplants.”
The lack of suitable tissue culture models makes studying iciHHV-6 challenging. That’s why Izumiya’s team is developing cell models using induced pluripotent stem cells, which can become any cell type in the body. They use 32 iciHHV-6 patient samples and generate stem cells. The team plans to differentiate cells into neurons and blood cells, with which they will learn when, where, and how iciHHV-6 start to replicate.
This work is in collaboration with Tetsushi Yoshikawa, chair of the Department of Pediatrics at Fujita Medical University in Japan. The study is supported by the National Institute of Allergy and Infectious Diseases.
Izumiya’s research is paving the way for potential new treatments for herpesviruses and cancer. By uncovering the secrets of herpesvirus, Izumiya and his team might use the genius strategies of one pathogen to fight another disease. Their work underscores the importance of basic medical research.
Related readings: