Izumiya Research Lab
Yoshihiro Izumiya, D.V.M., Ph.D.

Professor/Professor in Residence
Department of Dermatology
UC Davis School of Medicine
Research III
4645 Second Ave.
Sacramento, CA 95817
Phone: 916-734-7842
Yoshihiro Izumiya received his undergraduate degree in Veterinary Medicine at Kitasato University in Aomori, Japan in 1997, followed by a Ph.D. degree in Veterinary Microbiology in 2001 at the University of Tokyo in Tokyo, Japan. Between 1999 and 2001, Izumiya was a fellow of the Japanese Society for the Promotion of Science.
After Izumiya received his Ph.D. degree, he moved to the U.S. and expanded his research from animals to humans as a postdoctoral fellow in the UC Davis School of Medicine. As a postdoctoral fellow, he received an award for Excellence in Postdoctoral Research from UC Davis Health. In 2005, Izumiya was promoted to the title of Assistant Research Biochemist in the Department of Biochemistry and Molecular Medicine. In 2009, he joined the UC Davis Health Department of Dermatology and was promoted to Assistant Professor.
In his time at UC Davis Health, Izumiya was awarded multiple research funds, including a postdoctoral fellowship and IDEA award from the California HIV/AIDS Research Program, a Health Science System Award and a Translational Research Grant from the UC Davis Clinical and Translational Science Center (CTSC). Other research support includes grants from the United States Department of Agriculture (USDA) and National Institutes of Health (NIH). He also serves as a reviewer for virology-related journals such as the Journal of Virology, Virology and NIH Viral B study section.

 |
Yoshihiro Izumiya, D.V.M., Ph.D. Professor/Professor in Residence Research interests — Gene regulation of viruses such as Kaposi’s Sarcoma-Associated Herpesvirus |
 |
Mel Cambell, Ph.D. Assistant Project Scientist |
 |
Chie Izumiya, D.V.M. Assistant Project Scientist |
 |
Kevin Kim |
Not pictured:
Kazushi Nakano, Ph.D.
Visiting Assistant Researcher
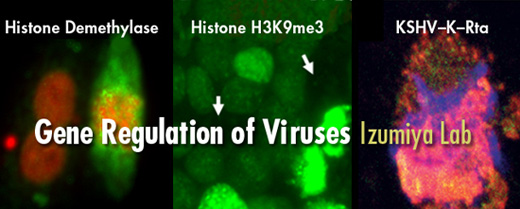
Yoshihiro Izumiya's Research Activities
Yoshihiro Izumiya, D.V.M., Ph.D., and his laboratory team study how genes are regulated through epigenetic mechanisms, using herpesvirus reactivation as a model system. While many viruses cause acute infections and are cleared by the immune system, herpesviruses establish lifelong latent infections. During latency, the virus hides by silencing its genes, but external triggers can reactivate it, switching on hundreds of genes at once. This makes herpesvirus an excellent model for studying how chromatin transitions from a “silent” to an “active” state in the genome.
Izumiya's research focuses on the epigenetic components of chromatin — both viral and host factors — that control how chromatin states are established, maintained and remodeled during viral reactivation. Since herpesviruses rely on host proteins for replication, their long co-evolution with humans also makes viral proteins valuable tools for drug discovery. By studying how viruses hijack host protein functions, Izumiya’s team aims to “reverse-hijack” these mechanisms to regulate host proteins for therapeutic purposes.
In collaboration with the biotech start-up VGN Bio, Inc., the lab is also developing anti-cancer therapies. These include antibody-conjugated drugs designed to deliver peptides identified through virology research. Beyond virology, the team’s findings have broad implications for cancer, development and aging, where epigenetic regulation plays a key role.
Selected Research Publications
Progress Reports (2018-2022)
Kumar A, Lyu Y, Yanagihashi Y, Chantarasrivong C, Majerciak V, Salemi M, Wang KH, Chuang F, Davis RR, Tepper CG, Nakano K, Izumiya C, Shimoda M, Nakajima KI, Merleev A, Zheng ZM, Campbell M, & Izumiya Y* (2022). KSHV episome tethering sites on host chromosomes and regulation of latency-lytic switch by CHD4. Cell Reports 39(6):110788. PMID: 35545047
Merleev A, Le ST, Alexanian C, Toussi A, Xie Y, Marusina AI, Watkins SM, Patel F, Billi AC, Wiedemann J, Izumiya Y, Kumar A, Uppala R, Kahlenberg JM, Liu FT, Adamopoulos IE, Wang EA, Ma C, Cheng MY, Xiong H, Kirane A, Luxardi G, Andersen B, Tsoi LC, Lebrilla CB, Gudjonsson JE, Maverakis E (2022). Biogeographic and disease-specific alterations in epidermal lipid composition and single cell analysis of acral keratinocytes. JCI Insight PMID: 35900871
Campbell M, Chantarasrivong C, Yanagihashi Y, Inagaki T, Davis RR, Nakano K, Kumar A, Tepper CG, & Izumiya Y* (2022). KSHV topologically associating domains in latent and reactivated viral chromatin. Journal of Virology 96(14): e0056522. PMID: 35867573
Merleev A, Ji-Xu A, Toussi A, Tsoi LC, Le ST, Luxardi G, Xing X, Wasikowski R, Liakos W, Brüggen MC, Elder JT, Adamopoulos IE, Izumiya Y, Riera-Leal A, Li Q, Kuzminykh NY, Kirane A, Marusina AI, Gudjonsson JE, Maverakis E (2022). Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a psoriasis susceptibility locus that is negatively related to IL36G. JCI Insight PMID: 35862195
Shimoda M, Lyu Y, Wang KH, Kumar A, Miura H, Meckler JF, Davis RR, Chantarasrivong C, Izumiya C, Tepper CG, Nakajima KI, Tuscano J, Barisone G, & Izumiya Y* (2021). KSHV transactivator-derived small peptide traps coactivators to attenuate MYC and inhibits leukemia and lymphoma cell growth. Communications Biology 4(1): 1330 PMID: 34857874
Kumar A, Salemi M, Bhullar R, Guevara-Plunkett S, Lyu Y, Wang KH, Izumiya C, Campbell M, Nakajima KI, & Izumiya Y* (2021). Proximity Biotin Labeling Reveals Kaposi’s Sarcoma-Associated Herpesvirus Interferon Regulatory Factor Networks. Journal of Virology 95 (9): e02049-20 PMID: 33597212
Liao Y, Lupiani B, Izumiya Y, & Reddy SM (2021). Marek’s disease virus Meq oncoprotein interacts with chicken HDAC 1 and 2 and mediates their degradation via proteasome dependent pathway. Scientific Reports 1; 637 PMID: 33437016
Nakajima KI, Guevara-Plunkett S, Chuang F, Wang KH, Lyu Y, Kumar A, Luxardi G, Izumiya C, Soulika A, Campbell M, & Izumiya Y* (2020). Rainbow-KSHV revealed heterogenic replication with dynamic gene expression. Journal of Virology 94 (8). e01565-19 PMID: 31969436
Campbell M, & Izumiya Y* (2020). PAN RNA: Transcription exhaust from a viral engine. Journal of Biomedical Science 27 (1): 41 PMID: 32143650
Liao Y, Lupiani B, Bajwa K, Khan OA, Izumiya Y, & Reddy SM (2020). Role of Marek’s Disease Virus (MDV)-Encoded US3 Serine/Threonine Protein Kinase in Regulating MDV Meq and Cellular CREB Phosphorylation. Journal of Virology 94 (17): e00892-20 PMID: 32581093
Zhou F, Liu X, Gao L, Zhou X, Cao Q, Niu L, Wang J, Zuo D, Li X, Yang Y, Hu M, Yu Y, Tang R, Lee BH, Choi BW, Wang Y, Izumiya Y, Xue M, Zheng K, & Gao D. (2019). HIV-1 Tat enhances purinergic P2Y4 receptor signaling to mediate inflammatory cytokine production and neuronal damage via PI3K/Akt and ERK MAPK pathways. Journal of Neuroinflammation 16:71 PMID: 30947729
Chen CP, Chuang F, Izumiya Y*. Functional imaging of viral transcription factories using 3D fluorescence microscopy. J Vis Exp. 2018 Jan 18;(131 PMID: 29443057
Campbell M, Watanabe T, Nakano K, Davis RR, Lyu Y, Tepper CG, Durbin-Johnson B, Fujimuro M, Izumiya Y*. KSHV episomes reveal dynamic chromatin loop formation with domain-specific gene regulation. Nat Communs. 2018 Jan 4;9(1):49. PMID: 29302027
Book Chapter
Davis RR, Campbell M, Izumiya Y, & Tepper CG (2020). Capture Hi-C: Characterization of Chromatin Contacts. Epigenetic Methods, Chapter 21