Research at the Department of Dermatology
Pioneering Breakthroughs in Skin Health
In recent years, the UC Davis Health Department of Dermatology has significantly expanded its research portfolio, making meaningful contributions to both science and medicine. With the addition of new faculty and the growth of our programs, we are shaping the future of dermatologic discovery and care.
In 2024, our department ranked No. 21 nationwide in National Institutes of Health (NIH) grant funding among U.S. dermatology departments (i.e., Blue Ridge rankings), reflecting our strong commitment to advancing skin health through research.
By uniting basic science and clinical research efforts, we foster a dynamic environment where discoveries in the lab translate directly into better patient care and patient needs guide scientific inquiry. This collaboration accelerates new approaches to prevention, diagnosis and treatment.
Basic and Clinical Research Activities
Basic Research
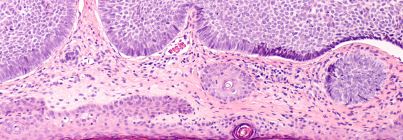
Basic science researchers within the Department of Dermatology focus on uncovering the fundamental biology of the skin and the mechanisms that drive skin disease. Their work spans skin development, inflammation, immunology, wound healing and cancer biology, laying the groundwork for new diagnostics and therapies. By investigating how skin functions at the cellular and molecular levels, they provide critical insights that guide translational breakthroughs.
Clinical Research
Our clinical researchers translate basic research discoveries into better treatments for patients. This team includes eight faculty investigators, supported by three clinical research fellows and five research coordinators. Together, they are leading 44 active Institutional Review Board (IRB)-approved studies addressing a wide spectrum of conditions such as atopic dermatitis, psoriasis, acne, chronic wounds, aphthous ulcers and skin cancers. These studies bring innovative treatments and novel therapies directly to patients, ensuring access to the most promising advances in dermatology.