Do chloride and pH regulation affect the heart muscle's ability to contract?
NIH grant funds novel cardiovascular research on cardiac chloride and pH regulation
UC Davis Health cardiovascular researchers are investigating the understudied but potentially critical connections between chloride and pH regulation and the heart muscle's ability to contract.
Xiaodong Zhang, associate adjunct professor of cardiovascular medicine, was awarded a four-year grant from the National Institutes of Health (NIH) to fund his “Cardiac chloride and pH regulation in health and disease” study.
“Heart disease is the leading cause of mortality in the United States and causes more deaths than all cancers combined,” Zhang said. “Coronary heart disease is accompanied by a major decline of local pH in the heart muscle. However, the mechanisms of pH regulation in heart cells remain incompletely understood.”
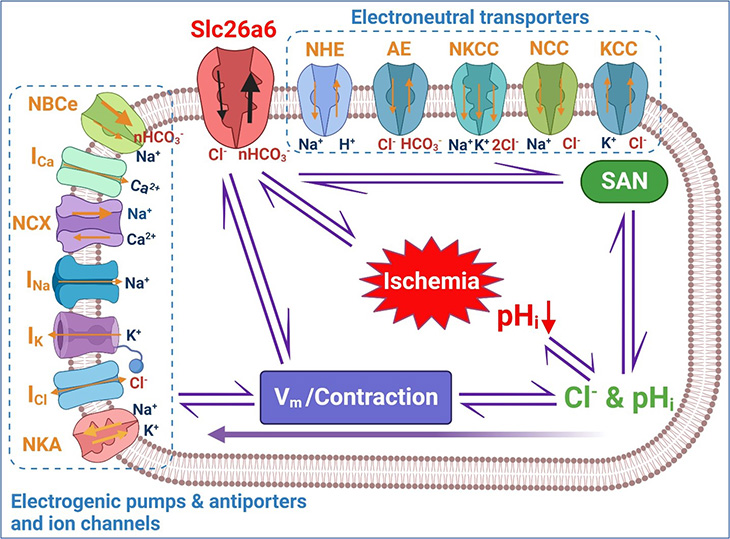
Links between cardiac chloride and pH regulation
Chloride ion is the most abundant anion, or negatively charged ion, in the human body. It plays an important role in cellular functions, including the regulation of electrical activity, pH and volume.
In skeletal muscles, chloride controls the excitability of muscle cells through its flux into and out of the cells. This flux stabilizes the cells' electrical potential, which prevents muscles from contracting abnormally.
Within the cardiac muscle (myocardium), chloride also contributes to the excitability of the cardiac cells, but the resting chloride conductance is not very high.
“This raised the question – how is chloride transported in the cardiomyocytes (cells responsible for the heart’s contraction) to maintain the relatively higher chloride concentration in the cardiac muscle cells?” Zhang asked.
Recently, Zhang and his team identified and cloned Slc26a6 (a membrane protein involved in transporting chloride, oxalate, sulfate and bicarbonate) as the most active and abundant chloride transporter in the cardiac muscle. Importantly, this membrane protein is an electrogenic chloride and bicarbonate exchanger.
The team documented through studies on mice that ablation of Slc26a6:
- Shortened the action potential of the cardiomyocytes
- Reduced heart rate
- Impaired cardiac function
- Increased the heart’s intracellular pH (pHi) – the measure of fluid acidity
“These results led us to believe that chloride is actively involved in regulating cardiac excitability and function,” Zhang added.
Additionally, the team examined whether the activity of Slc26a6 regulated heart rhythm and cardiac contractility via its pH-controlling mechanism. Because bicarbonate regulates the body's pH balance, the team predicted that the exchange of chloride and bicarbonate affected the pHi.
“Our recent findings showed that pHi in the cardiac cycle is not stable and experiences beat-to-beat changes,” Zhang explained. “Therefore, we need to understand how chloride and pH are dynamically coupled and regulated in the heart.”
Aim of clinical study
For their study, Zhang and his team will utilize multidisciplinary approaches to address three specific aims:
- Determine the regulatory mechanisms of Slc26a6 on cardiac pHi and function.
- Find the mechanistic roles of Slc26a6 in cardiac ischemia/reperfusion.
- Identify the roles and regulatory mechanisms of Slc26a6 in cardiac pacemaking activities.
The results should provide novel insights into the roles of Slc26a6 in cardiac pHi regulation, cardiac excitability and function under physiological and pathological conditions.
“We hope this study could lead to the development of new therapeutic strategies targeting chloride and pH regulation to prevent cellular damage from cardiac ischemia and arrhythmia,” Zhang said.