UC Davis Health offers highly accurate Alzheimer’s test
Ongoing improvements in testing may be game changer for early diagnosis of Alzheimer’s disease
Update May 16, 2025: The FDA has cleared the first blood test for use in diagnosing Alzheimer’s disease. The new test provides a less invasive option for diagnosing the disease. Learn more from the FDA news release.
What if physicians could diagnose Alzheimer’s disease before major symptoms appeared?
That’s possible with an FDA-approved test now offered at UC Davis Health. For people experiencing mild cognitive issues, the test helps their neurologists learn if the symptoms are related to Alzheimer’s.
A negative test can rule out Alzheimer's disease with 96.2% accuracy. A positive test can allow patients to receive a diagnosis and potentially access new drugs to slow the disease's progression while in a very early stage.
"This test is not new, but it is not widely offered," explained David Bissig, who helped make it available at UC Davis Health. Bissig is a neurologist and assistant professor in the Department of Neurology.
"We can now run our patients' tests here at UC Davis Health. Previously, we would need to send the tests to out-of-state reference laboratories,” Bissig said. “Offering it as UC Davis saves time and makes the test more cost-effective for our patients."
A primary care provider may eventually be able to screen for Alzheimer's disease as part of routine health maintenance — in the same way we screen for high cholesterol or diabetes. —David Bissig, Assistant Professor, Department of Neurology
Advances in Alzheimer’s testing allow earlier diagnosis
In Alzheimer's disease, the buildup of a specific protein — amyloid — is thought to impair signals between nerve cells in the brain.
In the past, physicians could only definitively diagnose Alzheimer's disease with a brain autopsy after the patient died.
But advances in testing now allow physicians to detect Alzheimer’s disease when patients experience symptoms.
One testing method used by neurologists, including at UC Davis Health, is an amyloid positron emission tomography (PET) brain scan. The scan can reveal brain changes indicative of Alzheimer's disease.
A second testing method now offered at UC Davis Health looks for unusual levels of specific proteins in cerebrospinal fluid, or CSF.
CSF is a clear liquid that bathes the brain and spinal cord. It is collected for testing with a spinal tap, also known as a lumbar puncture.
Alzheimer's test looks at specific proteins
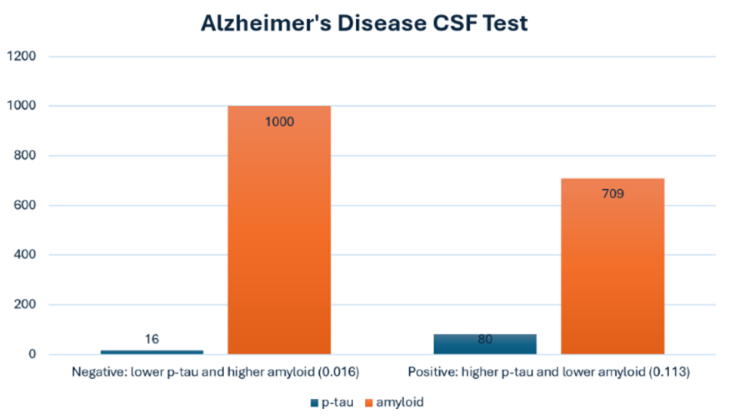
The Alzheimer’s CSF test examines the levels of three specific proteins, known as biomarkers, associated with amyloid buildup in the brain:
- 181 phosphorylated tau (p-tau181)
- 1-42 beta amyloid
- total tau proteins (t-tau)
These biomarkers are present in people without Alzheimer’s, according to Bissig.
“The ratio of p-tau to beta amyloid is the main thing we look at," Bissig explained.
People who do not have Alzheimer's disease have low p-tau because their neurons are not injured, according to Bissig.
“They also have high amyloid in their cerebrospinal fluid because it's being cleared from the brain,” Bissig explained. “This makes the ratio of p-tau divided by amyloid very low.”
People with Alzheimer’s disease have high p-tau, low amyloid
By comparison, people with Alzheimer's disease have high p-tau levels because their neurons are injured, causing the brain to leak tau proteins into the cerebrospinal fluid.
"People with Alzheimer's disease show lower amyloid levels because the amyloid is getting stuck in the brain and doesn't show up in the CSF,” Bissig said.
“Dividing that high p-tau by the low amyloid gives a big number, which is indicative of Alzheimer's disease," Bissig said.
Neurologists at UC Davis Health can order the CSF Alzheimer’s test for people with cognitive difficulties when they think Alzheimer's is plausible, and a positive test would change the medications they offer for treatment.
Center identifies high-impact tests and emerging threats
The new test was validated and deployed by UC Davis experts at the Center for Diagnostic Innovation Laboratory (CDxl).
The center includes clinicians, researchers and educators who identify emerging health threats that may impact the greater Sacramento region and California.
In addition to serving as a rapid response laboratory for emerging threats, CDxl also identifies high-impact tests and applications that can improve patient health.
“With new biomarker-based guidelines for Alzheimer’s disease and the unique FDA approval for tests to detect these biomarkers, it was an obvious choice to bring this test to fruition,” said Nam Tran, who spearheaded the effort. Tran is a professor in the Department of Pathology and Laboratory Medicine.
"This test was more complicated to bring up than others, even compared to our SARS-CoV-2 test in 2020,” added Tran. "Because we are an early adopter of this test, we did not have another test or facility to compare ours against."
The test was also more difficult because the biomarkers are very "sticky."
"This means the proteins can stick to the tube, potentially changing the results," Tran said. The team solved this challenge by using special test tubes during fluid collection.

Blood tests for Alzheimer's on the horizon
Blood tests for Alzheimer's disease — which would be significantly easier to administer than a CSF test — are still in development. However, researchers around the globe and some commercial laboratories are making rapid progress.
Tran and Bissig think blood tests will eventually be a game-changer for the disease.
CDxl is now working with Bissig and Charles DeCarli, a distinguished professor and co-director of the Alzheimer’s Disease Research Center, to save blood samples paired with CSF samples to validate these blood biomarkers at UC Davis Health.
"A primary care provider may eventually be able to screen for Alzheimer's disease as part of routine health maintenance — in the same way we screen for high cholesterol or diabetes," Bissig said.
But he also notes that while physicians can treat high cholesterol and diabetes, the treatment options for Alzheimer's disease are currently much more limited.
"New treatment options and medications need to be developed in parallel with earlier screening tests," Bissig said.




