UC Davis Health to develop in-utero therapy for Duchenne muscular dystrophy
California’s stem cell agency funds the potential gene editing DMD therapy
UC Davis Professor Aijun Wang and his team are collaborating with Murthy laboratory at UC Berkeley to develop a much-needed cure for Duchenne muscular dystrophy (DMD). They are designing a therapy to treat DMD before birth by editing the gene that encodes dystrophin, a key protein in stabilizing muscle fiber.
“We are developing a gene editing therapy that would allow pregnant mothers to give birth to children who are free from DMD,” said Wang, professor of surgery and biomedical engineering. Wang is the vice chair for translational research, innovation and entrepreneurship at the Department of Surgery and co-directs the Center for Surgical Bioengineering at UC Davis. He also leads the Wang Lab, a prime research hub in stem cell therapy and gene editing for early treatments of birth defects such as spina bifida.
This groundbreaking work is funded by a $2 million Quest Award from the California Institute for Regenerative Medicine (CIRM). The DISC-2 Quest Awards Program promotes the discovery of promising new stem cell-based and gene therapy technologies that could lead to broad use and improved patient care.

What is Duchenne Muscular Dystrophy (DMD)
Duchenne muscular dystrophy is a rare genetic disorder causing muscle loss and physical impairments in young people. It is characterized by muscle fiber degeneration due to a gene mutation that prevents the body from producing fully functional dystrophin.
Dystrophin protein plays a critical role in stabilizing the muscle membrane when muscles contract. Without it, muscle cells become damaged and weak.
The muscle weakness in DMD patients becomes increasingly noticeable between the ages of 3 and 5. By the age of 12, most patients require a wheelchair. By adolescence, the patient’s heart and breathing muscles weaken, leading to serious, life-threatening complications.
About Cure Duchenne Muscular Dystrophy (Cure DMD)
The team is developing a new technology called “Cure Duchenne Muscular Dystrophy (Cure DMD).” The hope is to treat DMD before birth by editing the genes that encode, or cause the production of, dystrophin. The plan is to edit these genes in the heart, diaphragm and limb muscles in utero and correct the DMD mutations before the onset of the disease.
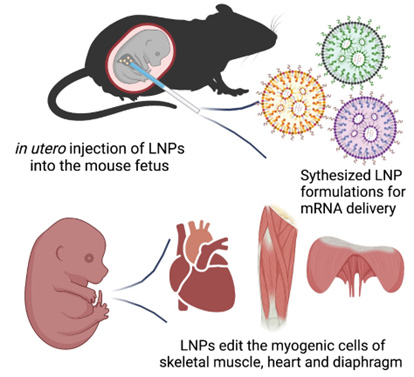
Cure DMD will be delivered via an in-utero injection to the fetus diagnosed with DMD. It will use a non-viral delivery method known as lipid nanoparticle to transfect, or introduce, cells using mRNA. This transfection causes the temporary expression of an enzyme that does the gene editing. The Wang and Murthy labs recently showed the feasibility of this approach in a study published in Bioactive Materials.
“Gene editing provides a permanent cure as it is a correction of the mutation,” Wang explained. “When the treatment is offered before birth, and if it is done right with mutation efficiently edited, one can prevent the disease from happening.”
Testing Cure DMD

The team will first test Cure DMD by giving an in-utero injection to a pregnant DMD mouse model. They will evaluate the safety of the treatment to the developing fetuses and pregnant female mice and monitor the edited mice for any birth defects.
The team will also test how well the formulation works in cells derived from human DMD patients.
“The gene editing efficiency will be a key performance endpoint that will determine the efficacy of Cure DMD,” said Niren Murthy, professor of bioengineering at the University of California at Berkeley and co-investigator on this project. The team anticipates achieving editing efficiency that will result in more than 5% of dystrophin expression.
How is Cure DMD different from other current therapies?
According to Wang, in-utero therapy holds unique advantages for accessing tissues that are hard to edit after birth. It would also allow the genetic disease to be corrected at an early developmental stage.
“A single injection would be sufficient for this therapy given that gene editing is permanent. The edited cells will proliferate and populate the organs naturally as fetal development continues in the womb,” Wang said.
The team also estimates that an injection in utero will be significantly more affordable than the current treatments for adult patients. Because of that, Cure DMD has the potential to be accessible to low-income patients and those in underdeveloped parts of the world.
We can potentially treat many patients before it's too late, just like in spina bifida.” —Aijun Wang, UC Davis professor of surgery and biomedical engineering
Currently, there is no non-viral gene editing treatment that can fully correct the mutation of the DMD gene or restore full-length dystrophin before birth. Cure DMD uses a non-viral gene editing mechanism. This gives it significant advantages over therapies now available and those currently in clinical trials.
Wang expects that the success of this study will open the door to treatments for many other diseases. Many patients with genetic disorders are not diagnosed or treated prenatally due to lack of treatment options. With the new gene editing and delivery technologies and more prenatal diagnoses, Wang thinks the field of treating genetic diseases will be very much changed.
“We can potentially treat many patients before it's too late, just like in spina bifida. If you can correct the spinal cord injury and prevent the neurons from dying, that's a big win and will make a long-lasting impact,” Wang said.

A dream team for Cure DMD
The study brings together top-notch experts at UC Davis. Craig McDonald and Lucas R. Smith serve as coinvestigators on this project.
McDonald is a professor and chair of the Department of Physical Medicine and Rehabilitation. He is an internationally recognized expert in the clinical management and rehabilitation of neuromuscular diseases, including muscular dystrophies, and the development of novel outcome measures for clinical trials.
Smith, an assistant professor in the Departments of Physical Medicine and Rehabilitation and Neurobiology, Physiology and Behavior, is a collagen and muscle characterization expert.
Other UC Davis collaborators include:
- Diana Farmer, distinguished professor and chair of the Department of Surgery and a world-renowned fetal surgeon
- Jan Nolta, director of the Stem Cell Program and the UC Davis Gene Therapy Center in the Institute for Regenerative Cures
- Nipavan Chiamvimonvat, professor and associate chief for research in the Division of Cardiovascular Medicine and a leading expert in cardiac physiology
- Herman Locsin Hedriana, medical director of prenatal diagnosis of Northern California and chief of the UC Davis Division of Maternal-Fetal Medicine. Hedriana is a leading expert in prenatal diagnosis and maternal-fetal medicine.
Related Stories: