Intellectual Developmental Disabilities
Research Findings
The UC Davis MIND Institute’s Intellectual and Developmental Disabilities Research Center (IDDRC) supports interdisciplinary research focused on understanding, treating and preventing the challenges associated with intellectual and developmental disabilities. Below are summaries of recent findings from UC Davis MIND Institute projects supported by the IDDRC.
-
By Gabriana La
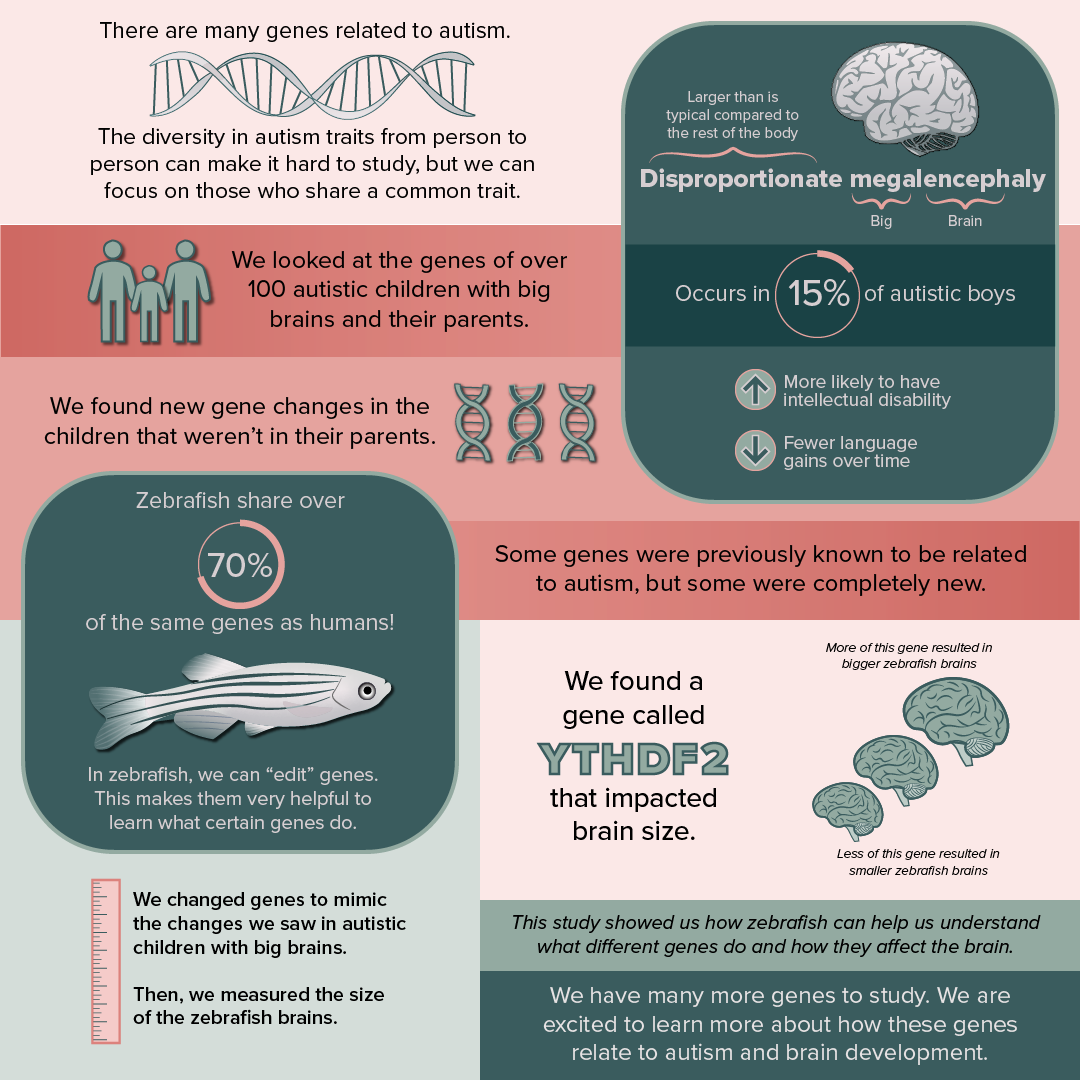
Autism, Big Brains, and Zebrafish?
Autism is a complex neurodevelopmental condition commonly associated with difficulties or differences in communication, social interactions, and repetitive behaviors. Researchers have found that autism is largely influenced by mutations in genes; however, the diversity of traits among autistic individuals and the many genes found to be associated with autism makes it a challenge to study.
To simplify the study of autism genetics, our MIND Institute lab led by Megan Dennis, an associate professor in the Department of Biochemistry and Molecular Medicine, focused on a subtype of autism associated with larger brains. This is termed disproportionate megalencephaly, with “mega-” meaning “big” and “-enceph-” referring to the brain. In autistic individuals, this enlarged brain often translates into language regression and intellectual disability, affecting about 15% of autistic boys. Through this study, we examined the DNA of autistic individuals with enlarged brains. This analysis identified mutations in over 100 genes in children not shared with their parents (so newly arising during the process of sperm or egg production). Among these genes, some were previously known to cause autism while others were completely novel.
With so many genes, we tested the functions of some of them using a “model organism” to understand if they changed brain size and development. Different types of animals are used to understand the neurobiology of autism. In our group, we use zebrafish. Believe it or not, despite hundreds of millions of years of evolution between fish and humans, we share over 70% of the same genes! Disrupting normal gene function is performed with molecular tools, like CRISPR, that can change the DNA and mimic the mutations we identify in autistic individuals. In “mutant” zebrafish, we can then test for changes in brain size after only a few days of their birth. This makes them a useful model for testing long lists of genes.
For our study, we mutated seven genes in zebrafish and identified one called YTHDF2 that impacted brain size. The gene produces a protein that impacts certain RNAs in the cell. Mutations leading to lower amounts of the gene resulted in smaller brains in zebrafish larvae, while higher amounts of the gene produced larger brains. The autistic individual with disproportionate megalencephaly also carried a mutation that produced more YTHDF2. Interestingly, another gene with similar functions affecting RNA called YTHDC1 was identified in a separate autistic individual with enlarged brain size.
These results provide an extensive list of many genes to explore and establish the use of zebrafish as a model for autism and brain size for further study. They also present an exciting new direction focused on genes related to RNA stability and transport in autism research.
-
By Andy Dakopolos
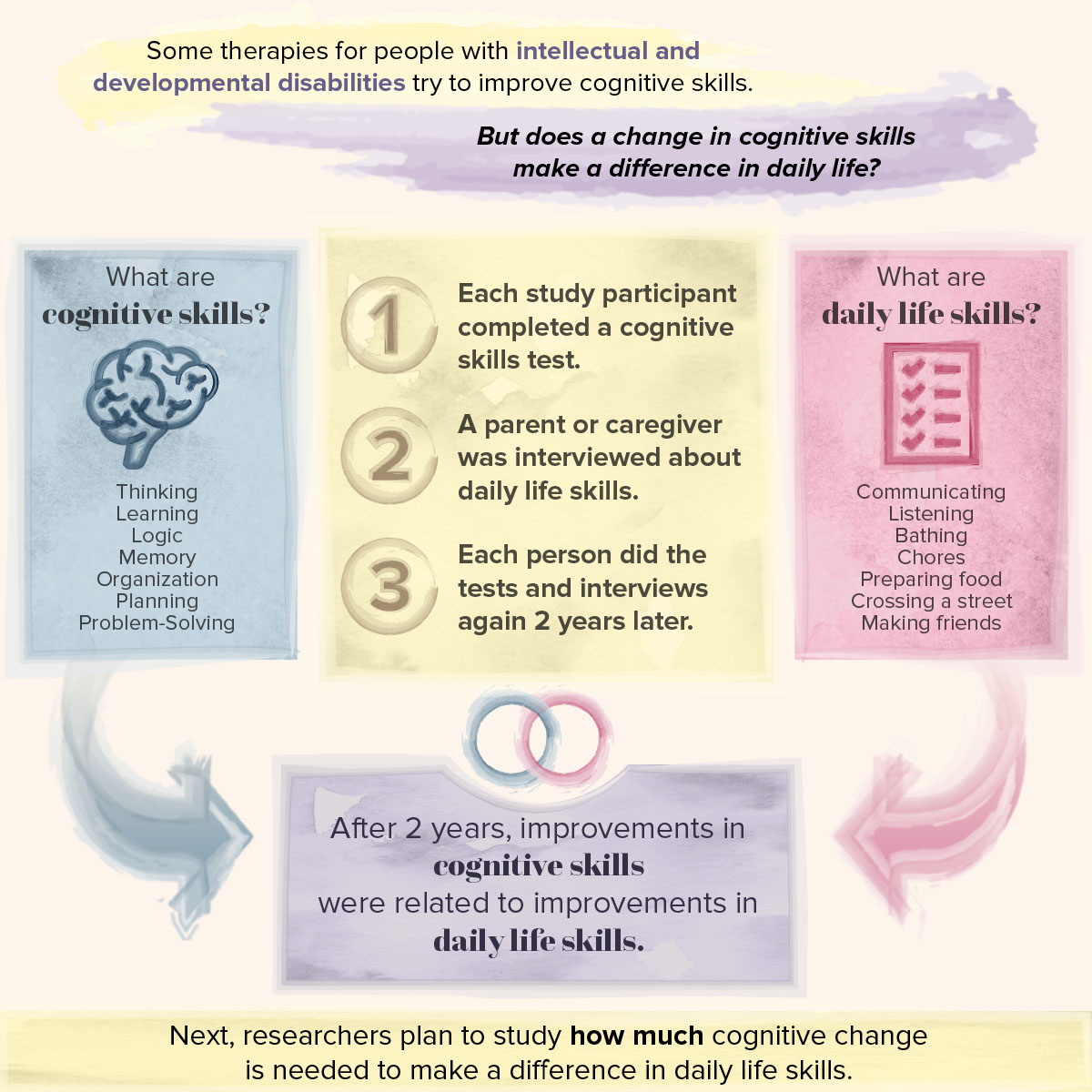
Measuring meaningful change in clinical trials
Promising clinical trials are currently testing treatments for individuals with intellectual and developmental disabilities aimed at improving cognitive skills. These skills include things like memory, planning and organization, flexibility, language and attention. To understand if interventions are effective, we need to know whether the cognitive changes we see in an individual are meaningful. In other words, do improvements in these kinds of cognitive skills actually impact everyday life?
This study answers this important question for people with intellectual and developmental disabilities. We tested cognitive skills in children, adolescents, and young adults with intellectual disabilities using an iPad app. Their parents or caregivers also completed an interview about how their child functions day-to-day. They were asked about things like communicating with others, listening skills, daily living skills like bathing independently, completing chores, preparing food, or crossing the street. They also answered questions about friendships, coping skills, and things they enjoy doing for fun. Two years later we invited everyone back to do the same cognitive tests on the iPad and conduct the parent or caregiver interviews.
In order to answer the question of whether changes in cognitive skills impact everyday life, we looked at whether the changes we measured in cognitive skills using the iPad app were related to changes reported during the interview over the two-year period. We found that when individuals with intellectual disabilities improve in their cognitive skills, this also leads to improvements in things like bathing, getting dressed, personal hygiene skills, cooking, and staying safe in the home and community.
This study is an important first step showing that cognitive improvements are directly linked to real improvements in the daily lives of individuals with intellectual disabilities. This work is not complete. The goal of our next project is to understand how big of a change in cognitive skills is needed in order to see real improvements in daily living skills. We are also currently using these approaches in clinical trials happening both at the MIND Institute and across the country.
-
By Christina Cabading
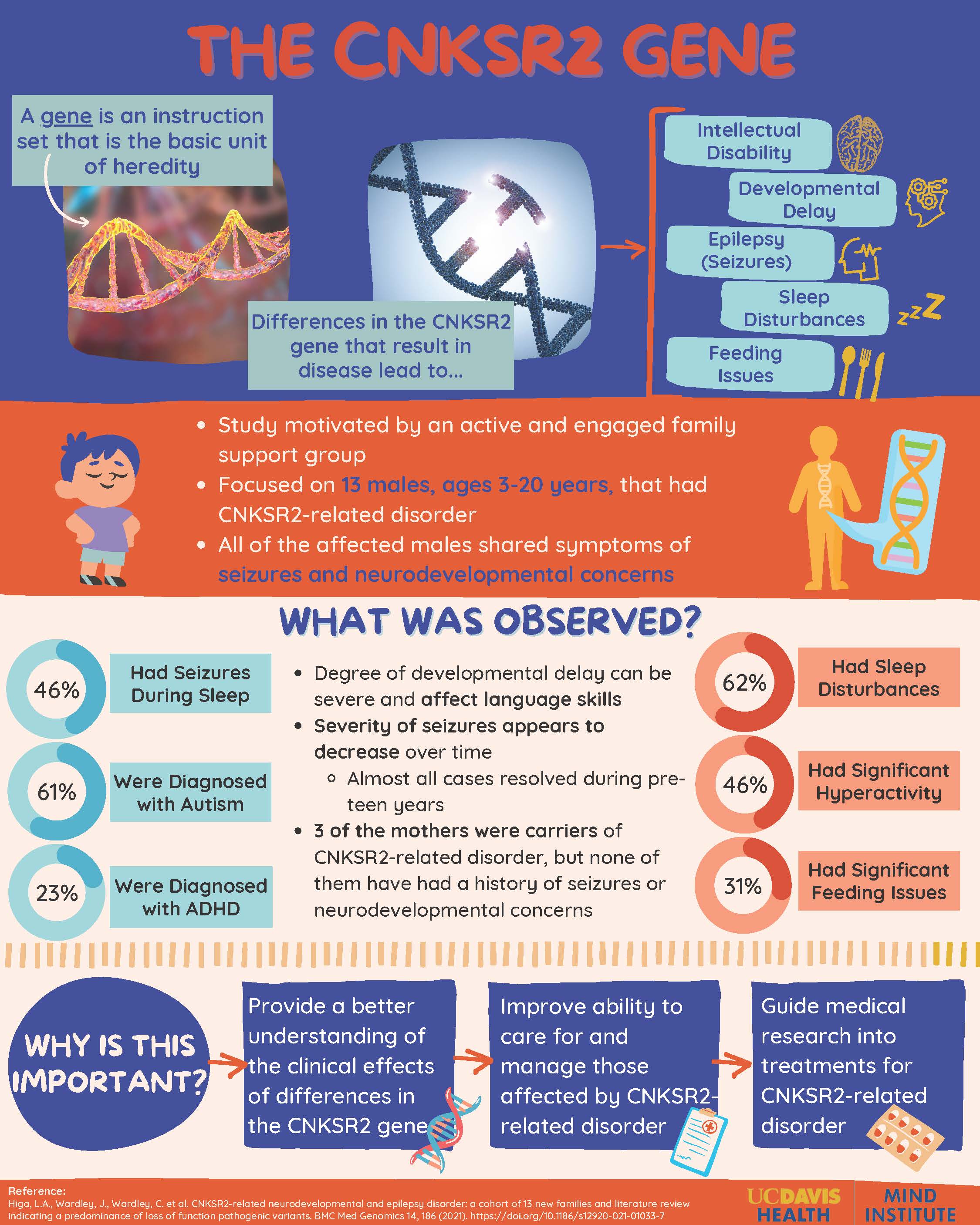
The CNKSR2 Gene
Genetic testing is important for researchers and families to explore the possible causes of neurodevelopmental conditions to help discover treatment and management in the future. Recently, it has been discovered that changes associated with loss of function to the CNKSR2 gene on the X chromosome can lead to intellectual disability, developmental delay, and seizures.
Motivated by the engagement and involvement of a genetics family support group, a 2021 MIND Institute study focused on more than a dozen new families around the globe that had mutations in CNKSR2. In these families, 13 males, ages 3-20 years, had the mutated gene. All of the males shared a common trait of seizures. Among the 13 individuals, 6 had seizures during sleep as shown through medical testing. However, it is important to note that the severity of these seizures decreased during childhood, and almost all cases were resolved in the pre-teen years. Along with the seizures, 8 of the 13 individuals were diagnosed with autism, and 3 of the 13 were diagnosed with ADHD.
Other medical issues associated with this disease were sleep disturbances, significant hyperactivity, and significant feeding issues. The mothers of the individuals with the gene were also tested. Three of the mothers were carriers of the CNKSR2 mutation, but none of them had a history of seizures or neurodevelopmental concerns.
One limitation of this study was the geographic location of the families. Because they lived in different countries around the world, variable information was collected. This was resolved by relying on clinical data collection on physical attributes of developmental delay, intellectual disability, seizures, etc. The overall purpose of the study was to be a starting point to identify additional families to gather more information about a larger number of individuals and how medical issues changes with age. The significant findings from this and future studies can help guide seizure management and be used to investigate treatment options for those affected by CNKSR2-related disorder.
-
By Sarah Bayoumi
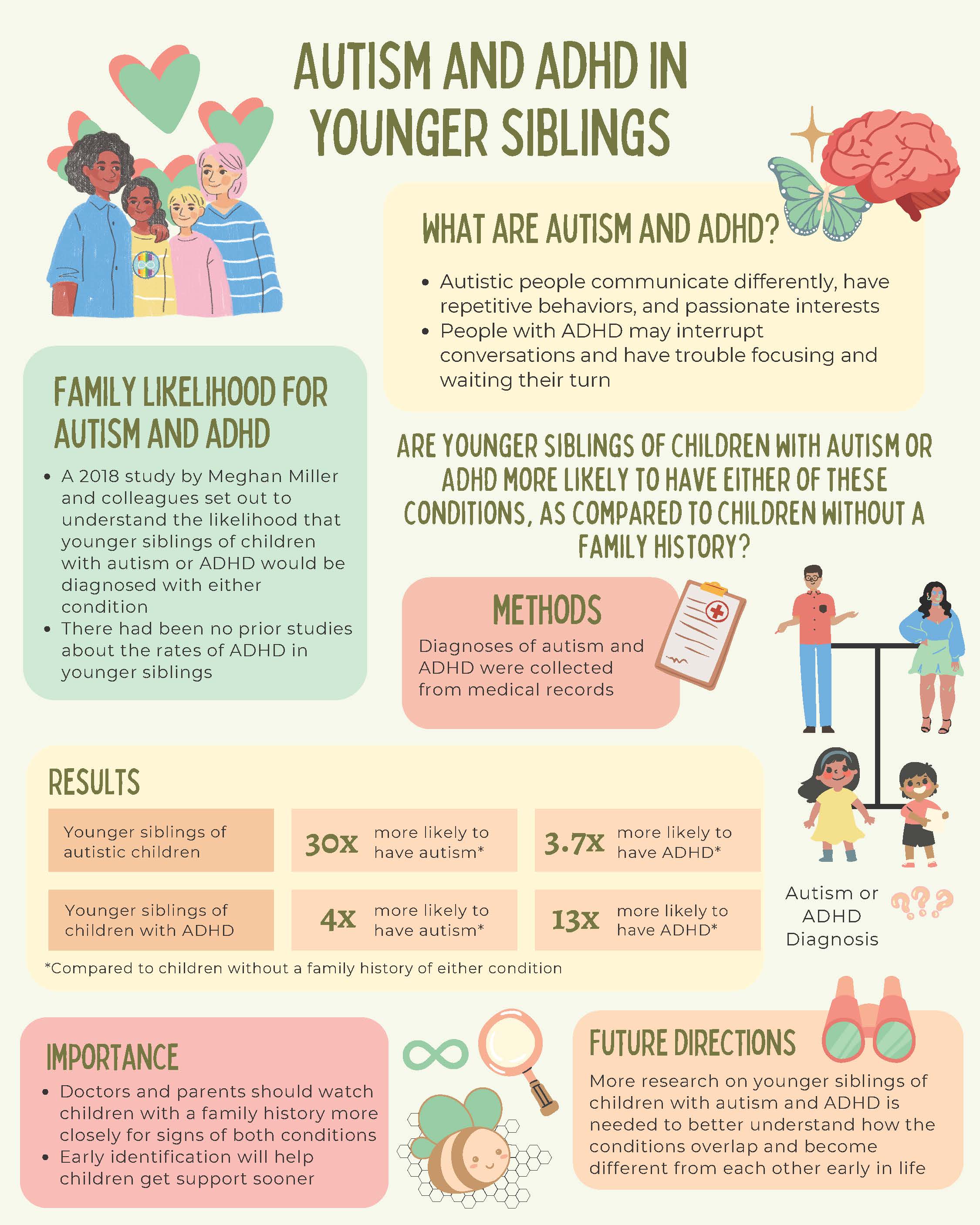
Autism and ADHD in Younger Siblings
Autism and attention-deficit/hyperactivity disorder (ADHD) are two common neurodevelopmental conditions that tend to run in families. Autistic people may have difficulty communicating and socially interacting, and they have repetitive behaviors and passionate interests. People with ADHD may interrupt conversations and have trouble with focusing, waiting their turn, and paying attention to detail. There are differences in the ways autism and ADHD affect people, but they share some genetic factors and similar traits such as difficulties with friendships and differences in skills like planning and organization. They also often co-occur.
A 2018 study by Meghan Miller, a clinical psychologist at the UC Davis MIND Institute, and colleagues set out to understand the likelihood that younger siblings of children with autism or ADHD would have either condition. There have been previous studies about the rates of autism in younger siblings of autistic children, but no similar studies in ADHD. No prior studies had looked at whether younger siblings of children with ADHD would have a higher chance of being diagnosed with autism, or if younger siblings of autistic children would have a higher chance of having ADHD. This study asked: Are younger siblings of children with autism or ADHD more likely to be diagnosed with either of these neurodevelopmental conditions compared to children without a family history of either condition? The researchers predicted that this would be the case because of the genetic overlap that is believed to exist between autism and ADHD. They looked at over 45,000 medical records to answer their question. So, what did they find?
In this study, children who had an autistic older sibling were about 30 times more likely to have autism and 3.7 times more likely to have ADHD compared to younger siblings of children without autism or ADHD. It was similar for ADHD: children who had an older sibling with ADHD were about 13 times more likely to have ADHD and 4 times more likely to have autism compared to children whose older siblings did not have autism or ADHD.
The findings are important for doctors and parents because they suggest that they should watch children with a family history more closely for signs of both neurodevelopmental conditions, not just for the condition that their older sibling has. Parents know their child best and can share any concerns with their doctor. Knowing this may help children get identified as having autism or ADHD earlier. This will help them get support sooner and have a better quality of life.More research on younger siblings of children with autism and ADHD is needed in order to better understand the links between the two, as well as how ADHD emerges in early life. Future studies will help us understand how autism and ADHD overlap and become different from each other early in life. This might help with earlier identification and earlier access to helpful supports. The LAAMB study at UC Davis is trying to understand this and is happening right now.
-
By Kendra Phillips
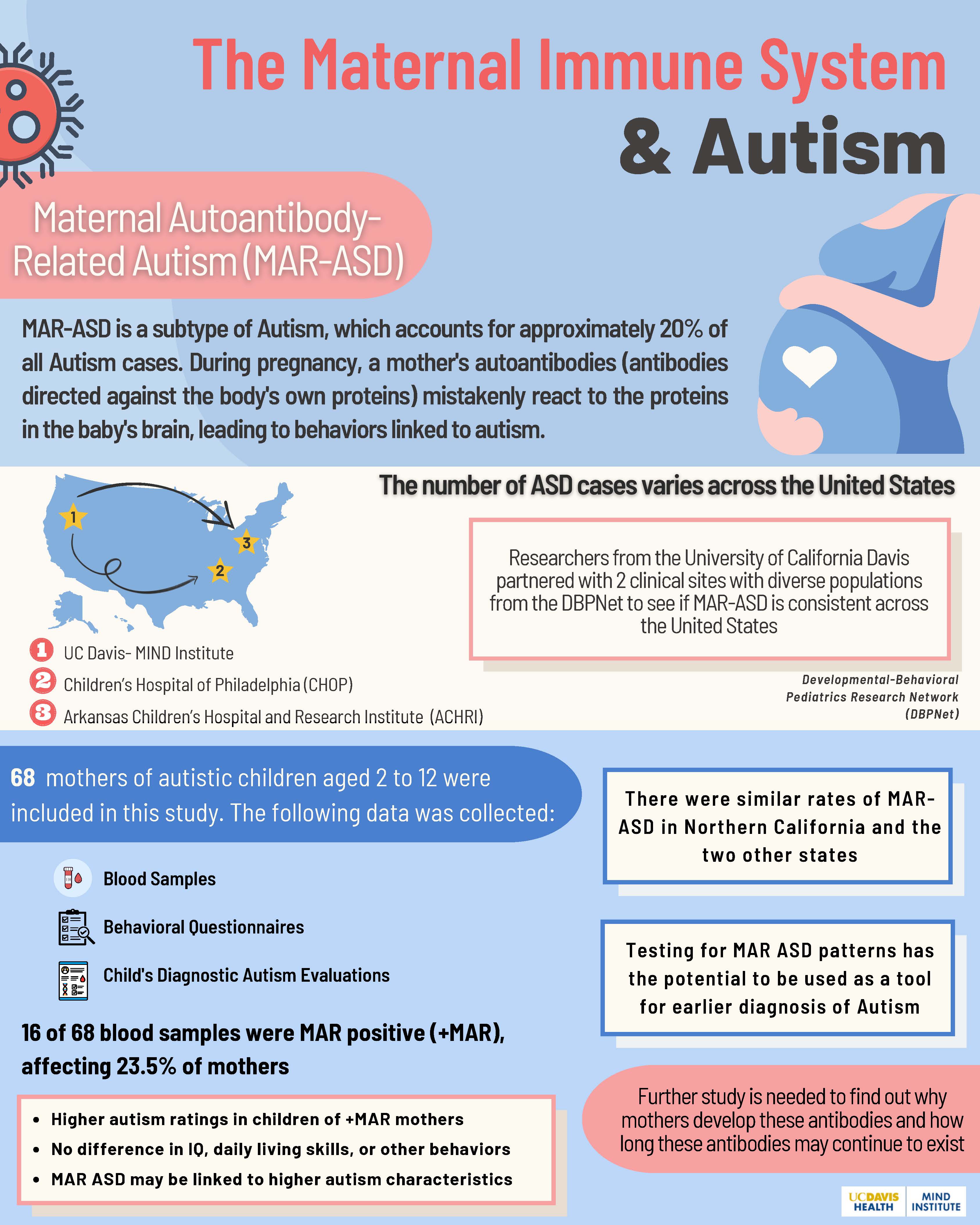
The Maternal Immune System and Autism
Autism is a neurodevelopmental condition that is diagnosed in 1 in 44 children in the United States. Causes of autism are unknown; however, researchers think that the immune system may play a role in some cases. The immune system makes proteins called antibodies to protect the body from bacteria and viruses that can cause infection. Sometimes, certain antibodies can mistakenly attack the body’s own tissues. These are called autoantibodies. Previous studies have found that some mothers of autistic children have certain autoantibodies that are almost never observed in mothers of non-autistic children. Researchers call this Maternal Autoantibody-Related Autism (MAR-ASD). The initial studies on MAR-ASD were conducted in Northern California and suggest that about 20% of autistic children are born to mothers with these autism-related autoantibodies, but it is not yet known whether MAR-ASD occurs at the same rate in other parts of the United States.
In a 2022 study, researchers from the University of California Davis partnered with two sites from the Developmental-Behavioral Pediatrics Research Network to determine if MAR-ASD is present outside of California. Sites included the Children's Hospital of Philadelphia and the Arkansas Children's Hospital and Research Institute. Sixty-eight mothers of autistic children aged 2 to 12 years old were included in this study. Mothers provided blood samples and completed behavioral questionnaires about their children. Data was collected from the child's diagnostic autism evaluations to assess autism symptoms.
Overall, 16 of 68, or 24%, of blood samples from the mothers contained autoantibodies associated with MAR-ASD. The rates were very similar across the two sites in Philadelphia and Arkansas . These rates were also very comparable to prior studies conducted in California. This shows that MAR-ASD is consistent across different populations throughout the United States. Another finding of this study was that autistic children of mothers with MAR-ASD autoantibodies had higher autism scores, or more intense autism behaviors, than autistic children whose mothers did not have MAR-ASD autoantibodies. There were, however, no differences across the two groups in IQ, daily living skills, or other behaviors were not found.
Ultimately, testing for MAR-ASD might determine the likelihood of a child having autism before characteristics are present. In the future, it has the potential to be used as a tool for earlier diagnosis of autism. Further study is needed to find why mothers develop these autoantibodies, how the autoantibodies affect the developing brain, and how long these antibodies may continue to exist.
-
By Catherine Gonzales
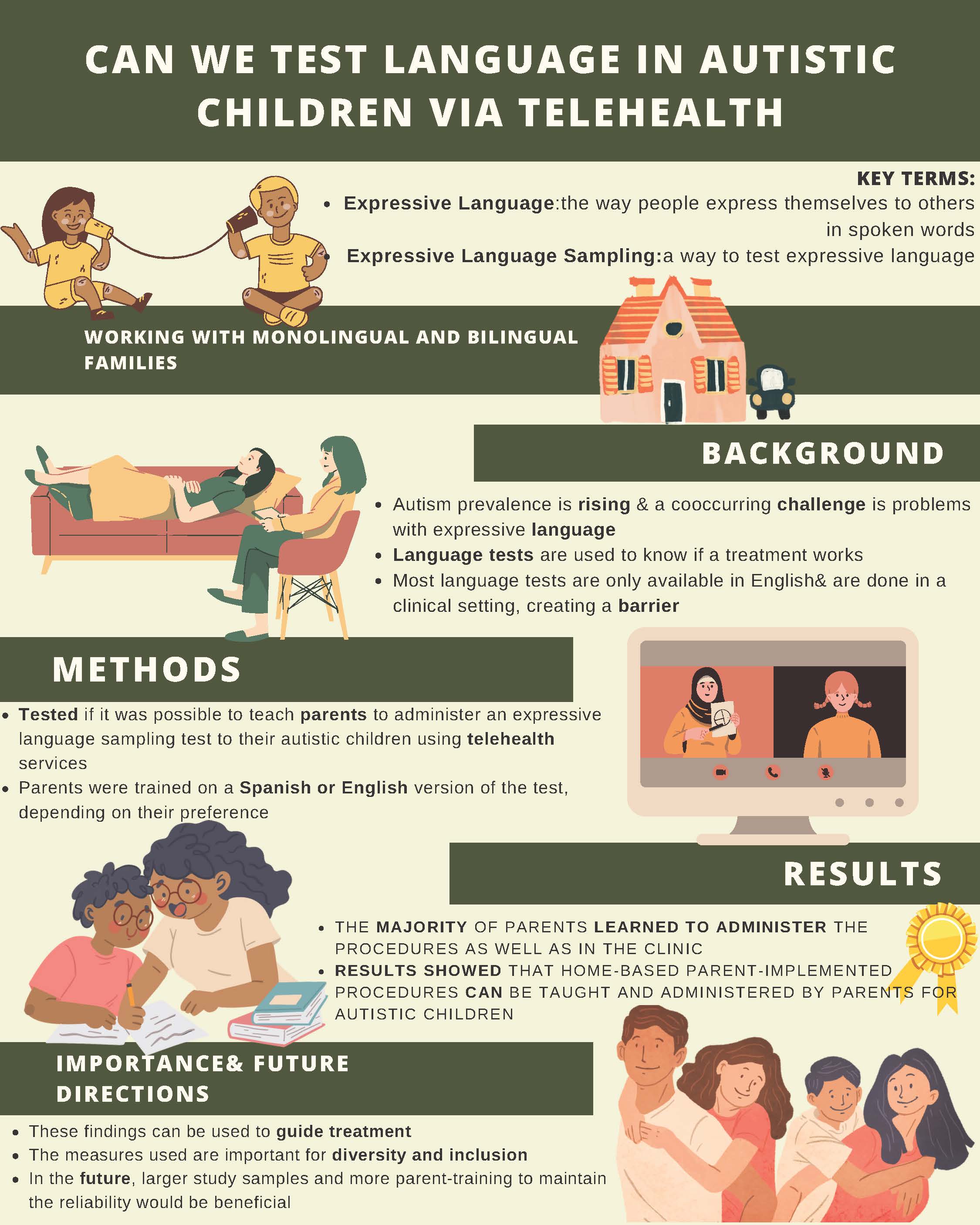
Can we test Language in Autistic Children via Telehealth
Expressive language is the way people communicate with others using spoken words. With the prevalence of autism rising and a major co-occurring challenge being expressive language skills, there is a need to be able to accurately measure those skills by collecting and analyzing expressive language samples to guide treatment. Until now, research on expressive language sampling validity has been limited to English speakers and carried out in clinical settings, which creates barriers.
The main goal of this study was to look into the possibility of teaching parents how to collect samples of their child’s expressive language for researchers to evaluate using telehealth services. This method gets rid of the need for families to travel to a clinical setting, allowing them to remain at home. In addition, the participants had the option of working with a provider who spoke the family’s preferred language. The study collected initial data on expressive language sampling for autistic children.
This study provided parent training in the parent's preferred language of either English or Spanish. The parents then used the training to collect expressive language samples in a standardized way from their children with autism. Twenty-two parent-child pairs participated from the MIND Institute's research participant registry. Eleven of these spoke English as a primary language, and 11 spoke Spanish. Of the people who completed the training, 16 out of 19 learned to run the procedures just as well as required in the clinic.
The results showed that home-based parent-implemented procedures can be taught and administered reliably by parents of autistic children. In the future, more studies with larger numbers of families would be beneficial, as well as more parent training to maintain the reliability of findings over time.
-
By Alejandro Perez
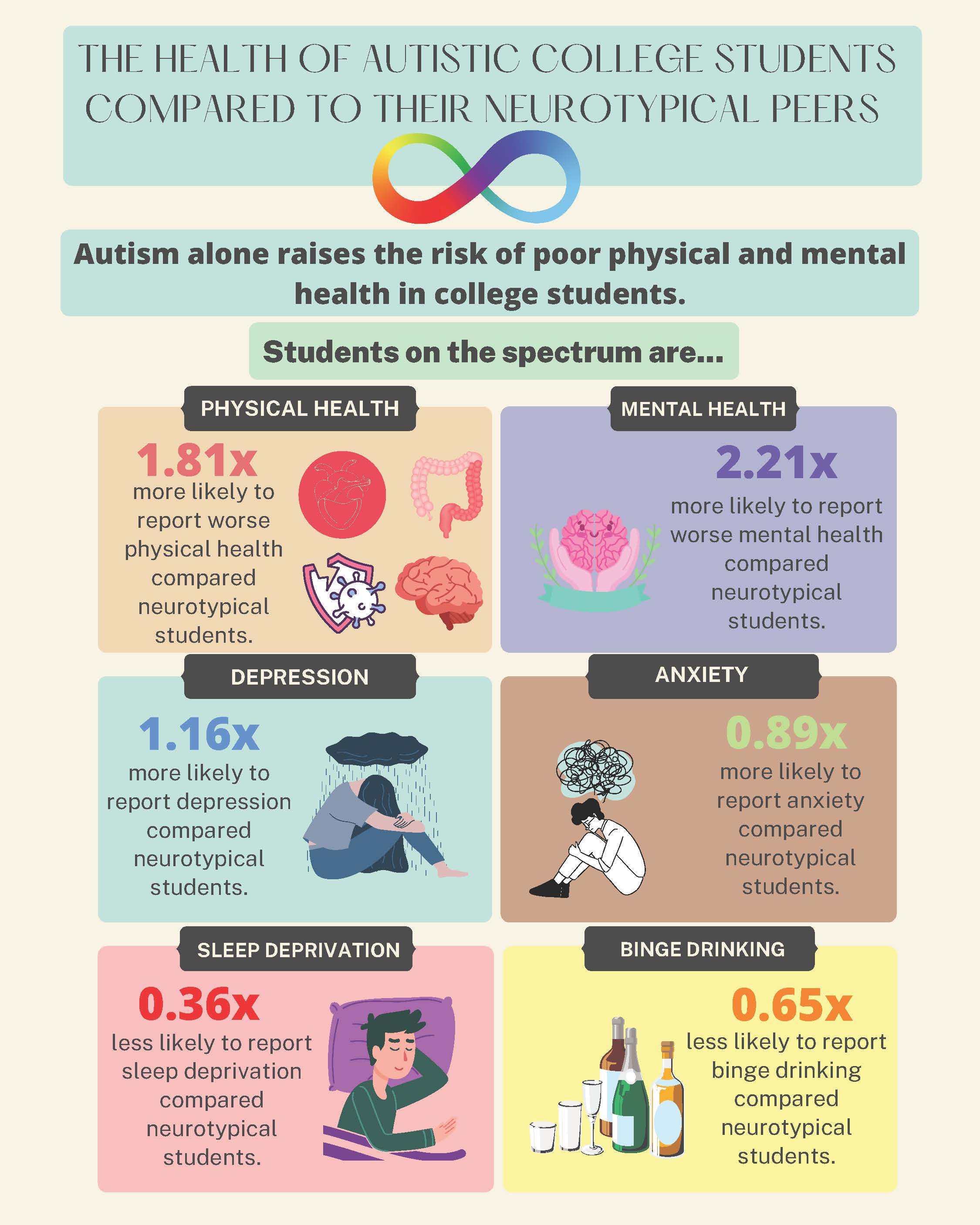
The Health of Autistic College Students Compared to their Neurotypical Peers
This study collected self-rated health data from 2820 autistic and non-autistic students at 14 colleges and universities with online surveys. They found that autistic college students reported worse physical and mental health, more depressive symptoms, and more anxiety symptoms than non-autistic students. On the flip side, autistic students also reported less sleep deprivation and binge drinking than other students.
This study is unique because it compares the physical and mental health of autistic students to their non-autistic peers and involves self-rated health. Self-reported physical and mental health was used because it predicts future morbidity and mortality more strongly than considering specific health conditions. The results suggest that these autistic students will continue to experience poor health. The higher levels of anxiety and depression found among autistic students are concerning for their overall health and academic success in college. These mental health issues decrease the likelihood of academic achievement.
The findings are significant since they were independent of underlying disabilities. This means autism alone raises the risk of poor physical and mental health in college students. The results show the need for more support services on college campuses to help the physical and mental health issues of autistic students.
-
By Meagan Ngyuen
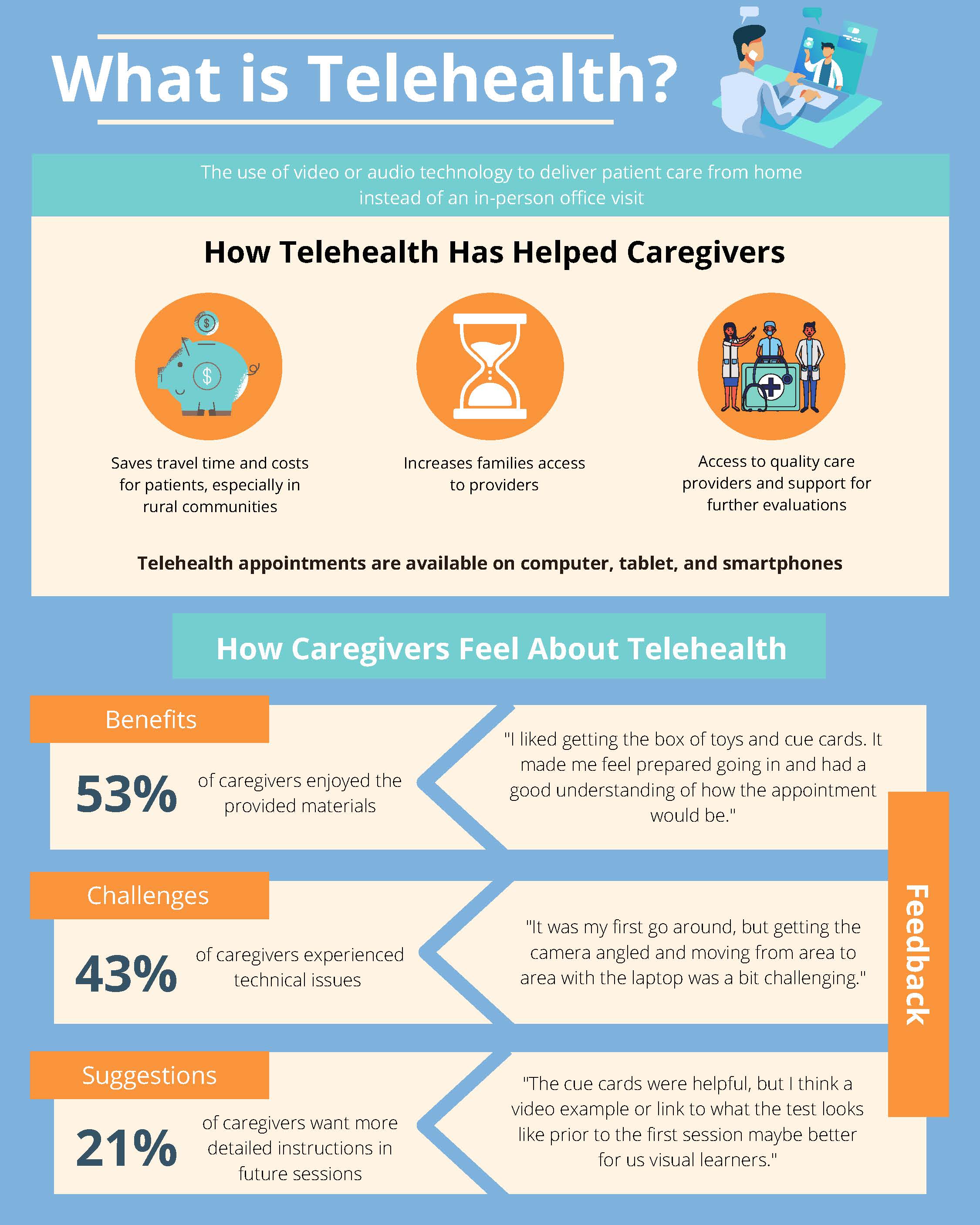
Benefits of Telehealth for Caregivers of Infants with Developmental Concerns
Autism is a developmental condition related to the brain and affects how a person may experience sounds, sights, and other experiences differently than others. The early signs of autism often appear in development over the first two years of life. Although clinicians have improved identifying and treating early signs of autism, there are still barriers that make it hard for families to find supports their children need. These include long waitlists for diagnostic assessments, time constraints, distance, and a lack of specialized providers in local community settings.
Telehealth is one way to increase families' access to assessments. In this study, we analyze the written feedback from caregivers who have participated in the TEDI study (Telehealth Evaluation of Development for Infants) to better understand the families’ experiences. By examining the pros and cons of telehealth-based assessments, we can make future evaluations better for families. We learned that most families liked the telehealth assessment. The main benefits families identified had to do with the convenience of not having to travel and having the materials they need provided by the study. Parents thought their infants behaved as they usually did during the telehealth sessions and found the technology easy to use.
We found that telehealth-based evaluations can be a great alternative for reliably assessing young infants and their behavior. One benefit of telehealth evaluations in our study is the more natural flow of observations between the parent-child interactions versus a more routine standardized assessment with an examiner. These findings suggest that this telehealth approach has the potential to provide increased access to services for rural and underserved communities. We hope in the near future that work can be done to learn more about the specific needs of families from more diverse, cultural, and socioeconomic backgrounds.
-
By Harjeet Mann
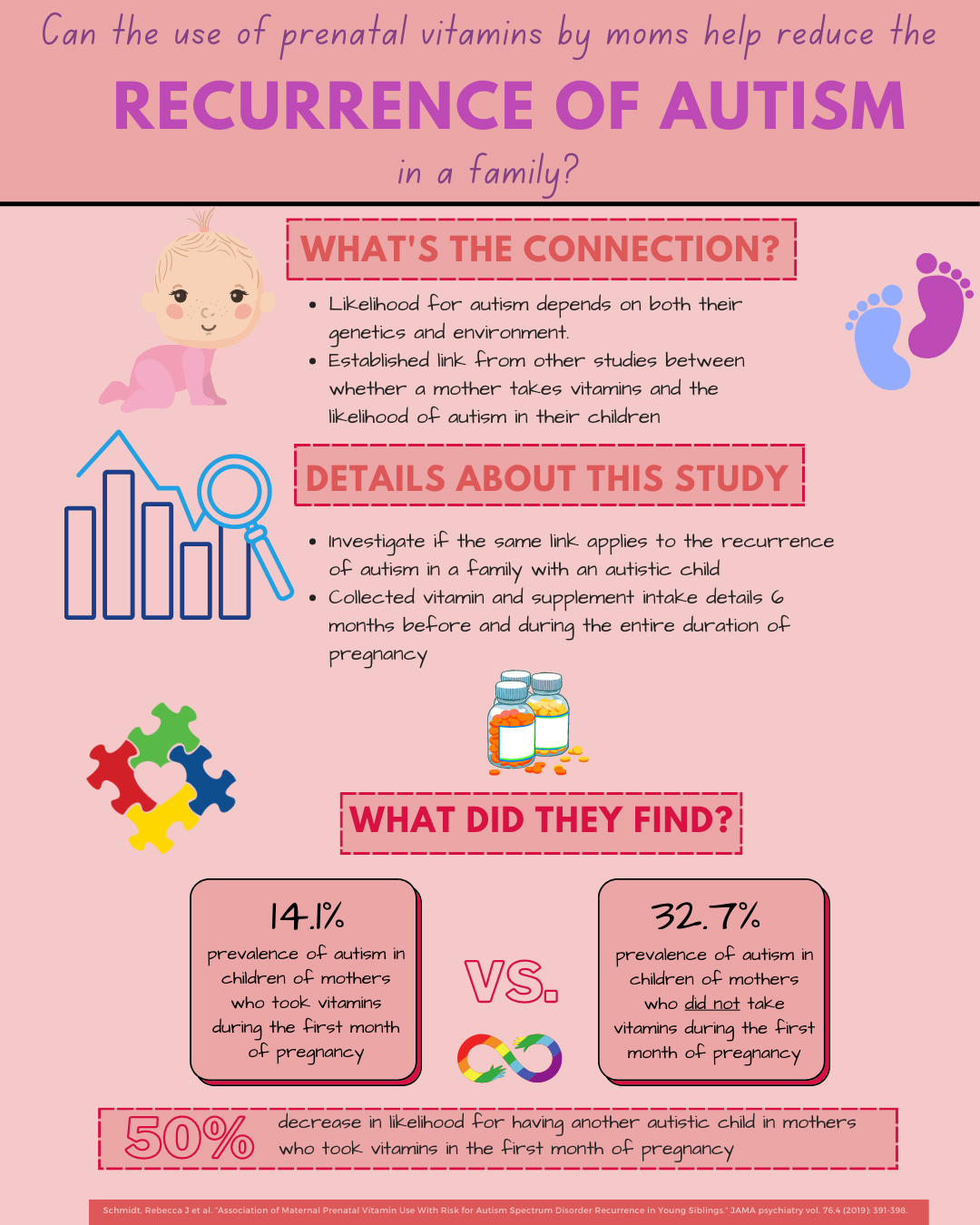
Does the use of Prenatal Vitamins Reduce the Likelihood of Autism?
Autism is diagnosed in about 1 in 44 children in America. The causes of autism are likely related to both genetic and environmental factors, and several studies have investigated factors that influence the likelihood of autism in children. Prior studies have shown an association between the intake of folic acid supplements in pregnant mothers and reduced likelihood for autism. With this in mind, this study investigated how the use of prenatal vitamins in pregnant mothers may reduce the likelihood of autism in the child. The main objective of this study was to determine if there is an association between the likelihood of autism in younger siblings of families who already have one autism child and the intake of prenatal vitamins. This study worked with participants from the UC Davis MARBLES study and collected data about lifestyle, demographics, environment, and medical information. They collected details about vitamin and supplement intake 6 months before pregnancy and during each month of pregnancy. The results of this study were that children born to moms who took vitamins during their first month of pregnancy were half as likely to be diagnosed with autism. The findings of this study are important because they add to growing evidence that prenatal folic acid vitamins are associated with a reduced likelihood of an autism diagnosis in children. Future studies can investigate other nutrients which affect the onset of autism and how the mother’s diet can contribute to decreased likelihood of autism in children.
-
By Paula Sullivan and Roy Ben-Shalom
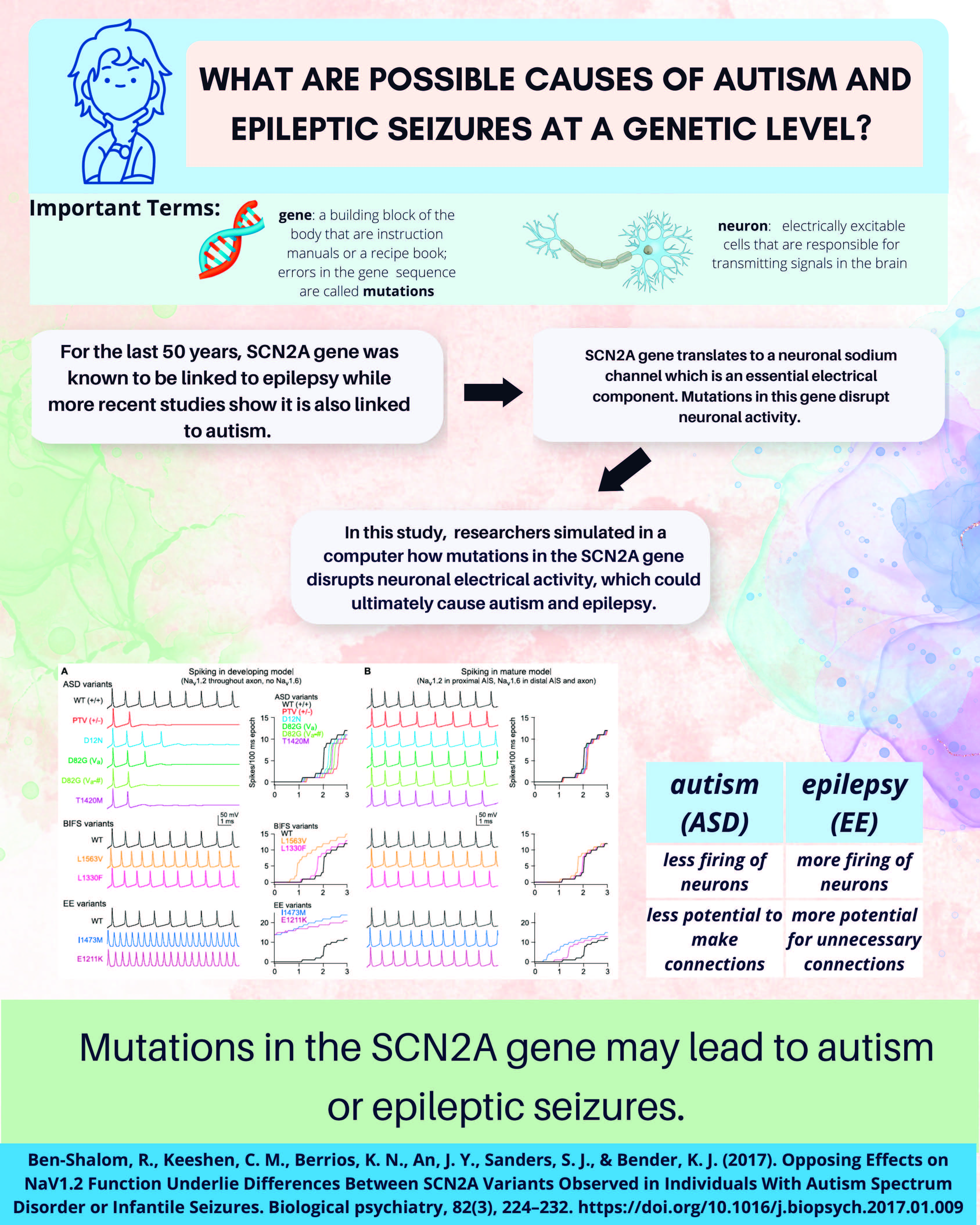
Mutations in the SCN2A gene may lead to Autism or Epileptic Seizures
Genes are the building blocks of our bodies. They provide an instruction manual for other biological factors to help our body grow and develop. Errors in genes are called mutations. Neurons are electrically excitable cells that are responsible for transmitting signals in the brain. For the last 30 years, the SCN2A gene was known to be linked to epilepsy. New genetic screening techniques found that the gene is also one of the most strongly implicated genes in autism as well.
The SCN2A gene codes for a specific type of sodium channel that is essential for a neuron’s electrical activity. Assistant Professor of Neurology Roy Ben-Shalom and colleagues wanted to know how different mutations in the same gene can cause such different conditions – autism in some cases and epileptic seizures in others. In the first of a series of two studies, they used a computer to simulate how mutations in the SCN2A gene disrupt neuronal electrical activity. They found that in epileptic seizures, there was more neuronal electrical activity, but in autism, there was less. From these results, they reasoned that changes in electrical activity during early development might affect brain development and lead to autism. In other words, less firing of neurons or less electrical activity in neurons may lead to autism.
Next, Ben-Shalom and his colleagues generated an autism mouse model with a mutation in the SCN2A gene. When they recorded from its neurons, they found that as the computer model predicted, these neurons fire much less than a mouse without the mutation. In their second study, they tackled the question of how a disruption of SCN2A sodium channels leads to reduced neuronal activity. To answer this, they looked at the dendritic part of the neuron, which is the part that receives input from neighboring neurons. They found that the input neurons were not as effective in exciting the dendrites of the neuron because of a lack of sodium channels. Less activity in the dendrites can lead to disrupted neuronal connections, similar to what has been observed in other autism-associated genes. These findings show how a mutation in the SCN2A gene leads to reduced sodium in the dendritic portion of the neuron, which in turn leads to altered neuronal networks. These findings may provide new insights into the underlying neuronal mechanisms of autism.
-
By Aubyn Stahmer
The California Autism Professional Training and Information Network (CAPTAIN)
It is important for teachers and other educators working with autistic individuals in public schools to be trained in intervention strategies that have been researched and shown to work. Using these evidence-based practices is required by federal legislation and leads to better outcomes for autistic students. The challenge is that educators don’t always have adequate training on how to implement evidence-based strategies.
The California Autism Professional Training and Information Network (CAPTAIN) is a state-wide network that aims to encourage the use of high-quality, evidence-based strategies in the education of autistic individuals throughout the state. CAPTAIN has over 200 trainers who work in schools to provide instruction to almost 20,000 educators and individualized coaching to over 6,500 educators each year. A recent study published in the journal Autism by Aubyn Stahmer and colleagues tested whether being trained by CAPTAIN helps educators use evidence-based strategies more effectively in their classrooms.
The study was conducted at UC Davis and San Diego State University. The researchers surveyed educators trained by CAPTAIN and those not trained by CAPTAIN about their use of evidence-based practices, then compared the answers.
They found that CAPTAIN-trained educators reported better attitudes toward using evidence-based practices, were more likely to use the strategies correctly and used them more often with students. They had more in-depth knowledge of their primary intervention strategy and better ratings of classroom quality than educators who did not get CAPTAIN training. These findings show great promise for CAPTAIN as a model to support statewide use of better evidence-based strategies for autism. More studies are needed to see how the use of these practices improves student outcomes.
-
By Derek Andrews
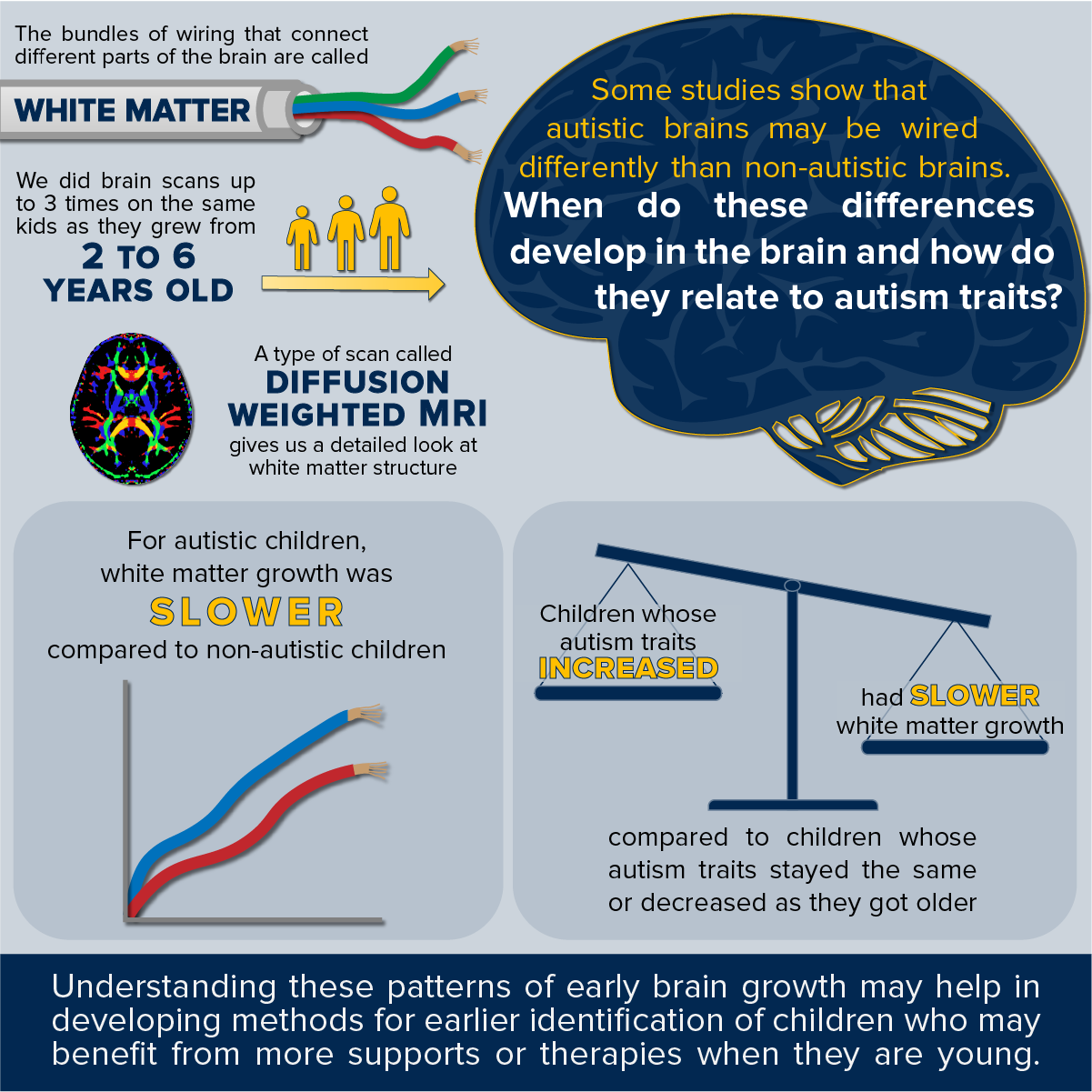
Alterations in Brain Wiring Associated with Changes in Autism Symptoms
My colleagues and I recently published a study that helps to explain differences in the development of the brain’s wiring in autism, and how these differences are associated with changes in autism symptoms across childhood.
Within the brain, white matter serves as connective tissue, “wiring” different parts of the brain together (like bundles of wires). These structural connections provide critical pathways for different brain regions to communicate with each other to produce a desired behavior. It is widely theorized that autistic characteristics, including social communication challenges and restricted repetitive behaviors arise from alterations in brain connectivity. A type of magnetic resonance imaging (MRI) scan called diffusion weighted MRI is particularly well suited to investigate the white matter pathways in the brain and has been utilized by several previous studies of autism. These studies have consistently found alterations in white matter structure in autism; however, little is known about when these differences develop.
This recent study leveraged diffusion weighted MRI scans from the UC Davis Autism Phenome Project, which was first initiated in 2006 by Professors David Amaral and Christine Wu Nordahl. This project continues to follow children at multiple points across childhood starting from around three years of age, near the time when an autism diagnosis is first possible. By acquiring multiple brain scans longitudinally across childhood, we were able to chart the developmental course of white matter pathways in autism compared to typical development. We found that in autism, white matter differences in these pathways emerge over early childhood due to slower white matter development and might not be present, or even have differences in the opposite direction closer to three years of age. Importantly, slower white matter development was found to be associated with a trajectory of increasing autism characteristics. This provides new evidence that white matter development may serve as an important indicator of the progression of autism.
We hope in the near future that measurements such as this can both identify children who would benefit from more intensive intervention and serve as a marker to determine the effectiveness of interventions. Accomplishing this would allow us to better tailor interventions for individual children and hopefully improve the quality of their lives.
-
By Monique Moore Hill
Screen time Associated with Increased Inattention and Hyperactivity
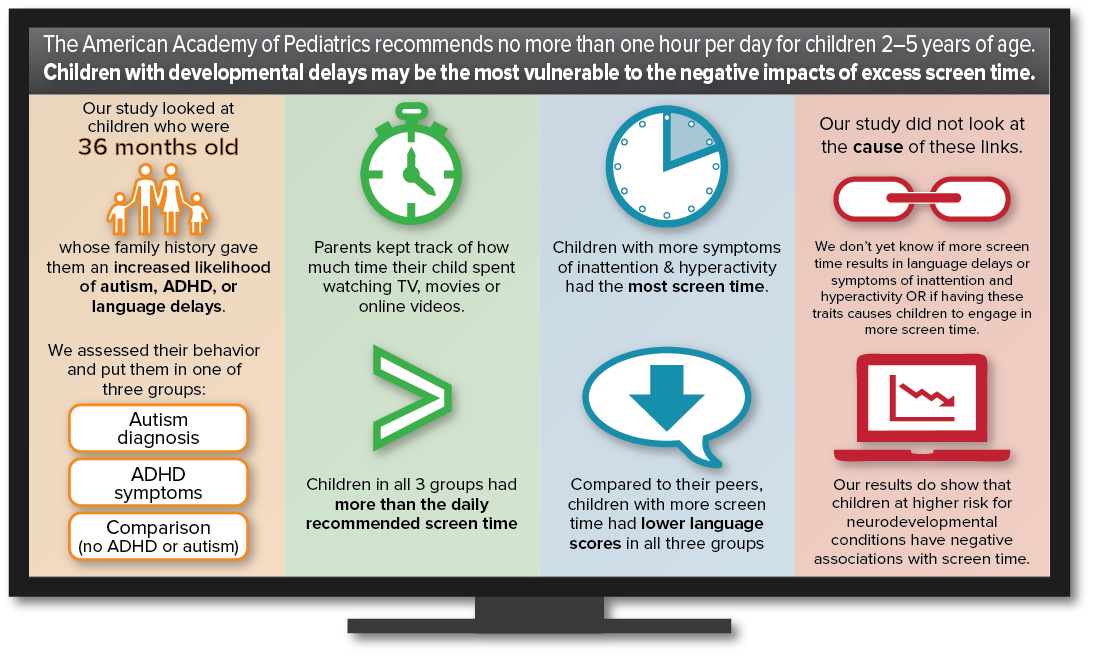
The American Academy of Pediatrics (AAP) recommends no independent screen time (i.e., digital media use) for children younger than age 2, and no more than one hour per day for children 2–5 years of age. These guidelines are based on research indicating that children who engage in less screen time generally have higher language and developmental scores.
Most prior screen time research has involved the general population. However, children with developmental delays and those with behavior problems may be the most vulnerable to the negative impacts of excess screen time. That’s because screen time may replace developmentally beneficial interactions.
A recent study in our lab examined screen time in 36-month-old children at increased likelihood for autism spectrum disorder (ASD), language delays, and attention deficit/hyperactivity disorder (ADHD) due to family history. The sample also included a comparison group of children with no family history of neurodevelopmental conditions.
Parents reported how much time their child spent watching television programs, movies, streamed media content or YouTube videos. Children received assessments in the laboratory to evaluate their behavior and language development. Based on these, children were grouped into three categories: ASD diagnosis group, elevated ADHD symptom group, and Comparison group (any children who did not meet criteria for the other two groups).
Results showed that, on average, children in all groups exceeded the daily recommended screen time guidelines of one hour per day. Children with increased symptoms of inattention and hyperactivity had the most screen time, with an average of 2.33 hours per day. In addition, more screen time was associated with lower expressive and receptive language scores in all groups, including the comparison group who showed no signs of either ADHD or ASD symptoms.
Because our study evaluated behavior at a single time point we cannot determine the cause of the associations. Some parents may wonder if allowing their child to watch television or videos at an early age may cause ASD or ADHD. A causal association between screen time, ASD and ADHD is not indicated in prior research or in our study. More research is needed to understand whether increased screen time contributes to language delays, inattention, and hyperactivity, or if children in these groups engage in more screen time for other reasons (e.g., preference for visual input, parental viewing habits, sleep habits, etc.).
Our results do indicate that the negative associations found between screen time, language development, and inattentive/hyperactive behaviors demonstrated in community samples of children in previous studies are also evident in children at risk for neurodevelopmental conditions. Our findings provide additional support for the AAP’s current recommendation to limit screen time in young children.
-
By Fereydoun Hormozdiari
Developing Innovative Methods to Study the Human Genome
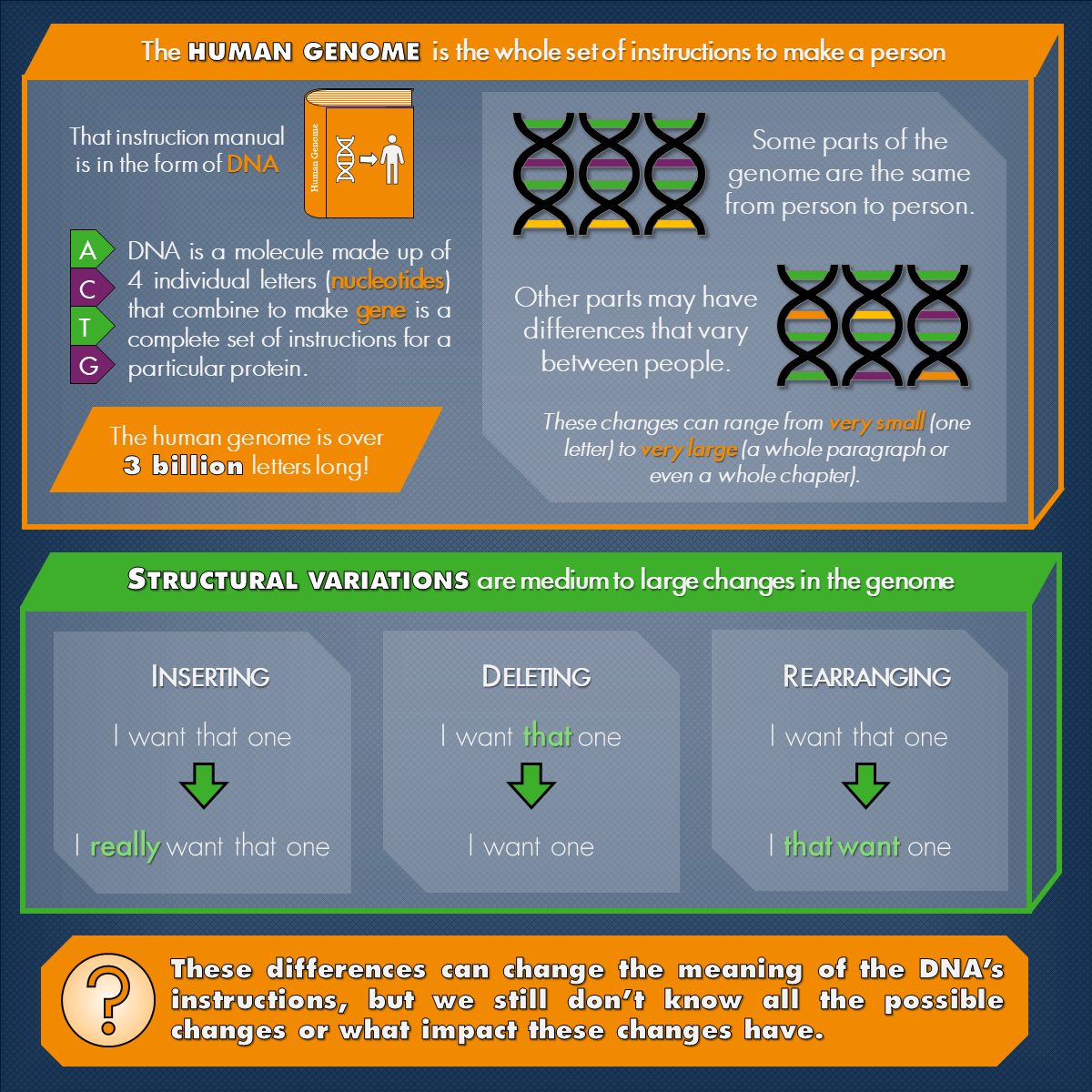
The human genome is the complete set of DNA that makes up a person. It consists of a long stretch of over 3 billion nucleotides that combine in a certain order to form thousands of genes. These genes, in turn, encode proteins, which are the building blocks of each cell. Genomes contain each person’s unique genetic code and are responsible for various complex traits and common diseases. Genetic variations, or mutations, are differences in these genetic codes that arise when the genome sequences are modified through small or large changes.
Structural variations (SVs) are defined as medium and large genomic rearrangements, involving more than 50 base pairs (sets of nucleotide) changes. These rearrangements to the genomic sequence may be in the form of insertions, deletions, or inversions of nucleotides. A growing body of evidence has shown that SVs in a person’s genome are a major contributing factor to diseases (such as cancer) as well as neurodevelopmental conditions (like autism). There are still many unknowns about SVs including their diversity, complexity, distribution in the population, and their exact impact in biology. These are some of the reasons that my lab is conducting a major project, funded by the National Science Foundation CAREER Award, to develop new computational methods to study SVs.
Recent advances in sequencing technology have allowed us to investigate the complexity of SVs and their direct contribution to different diseases or traits. To fully investigate these variants, we need accurate and efficient computational approaches to discover and characterize different types of complex SVs. The goal of our project is to develop novel methods to provide researchers with necessary tools to better capture the diversity of SVs and study their contribution to biology.
As part of this project, novel methods will be developed for efficient and accurate genotyping of any type of SV. These tools will be directly applicable to studying the impact of both common and rare SVs on neurodevelopmental conditions such as autism. To establish the utility of these methods, we will analyze publicly available whole-genome sequence data. This project will also achieve a broader impact by providing training opportunities for both undergraduate and graduate students interested in computational genomics. The results of the projects will be available at www.hormozdiarilab.org.
-
By Meagan Talbott
Developing digital telehealth assessments for autism
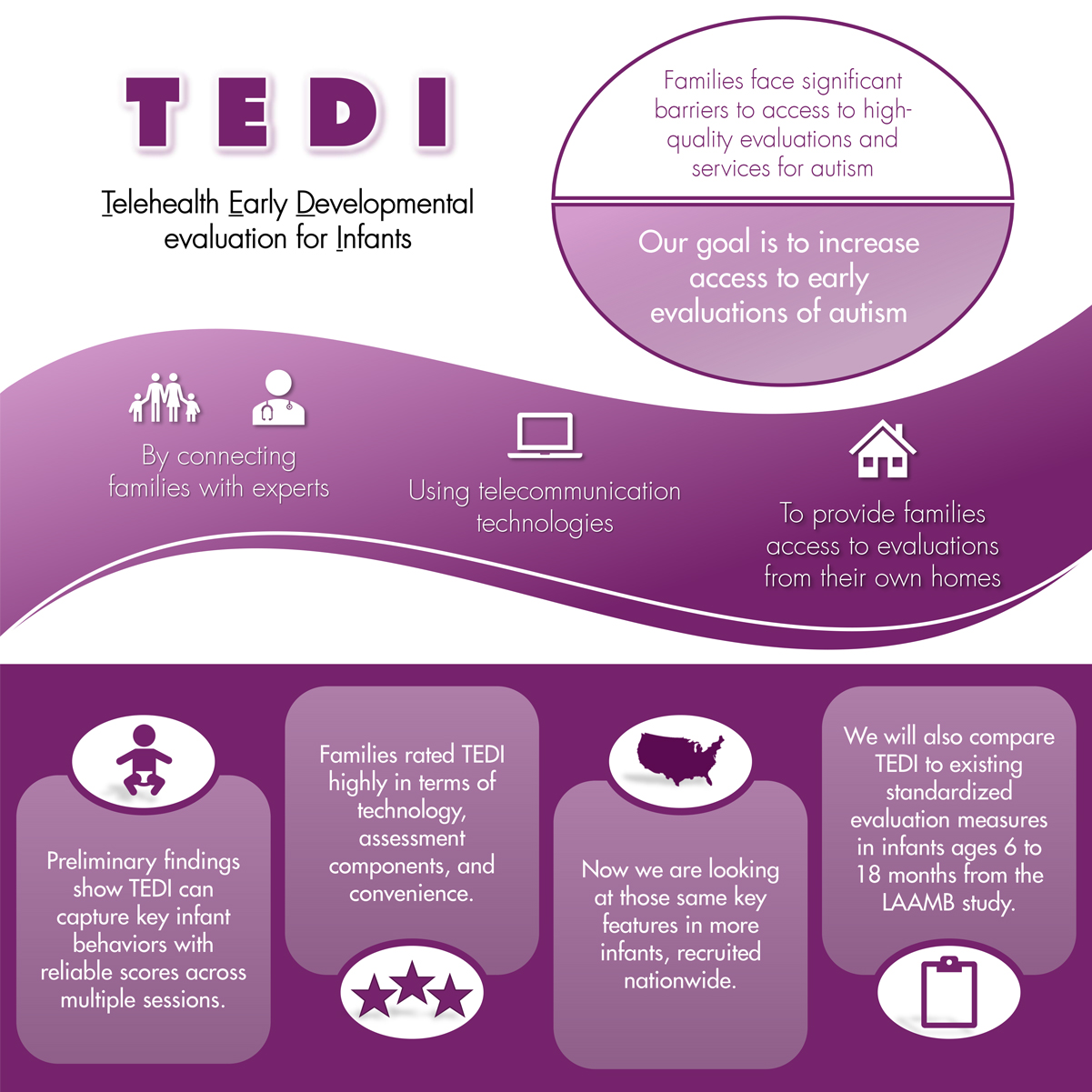
Over the last few decades, we have learned a great deal about the early development of autism. For example, we know that for most children on the spectrum, the signs of autism emerge over the first few years of life. Despite advances in early identification, there remain significant barriers to families’ access to high-quality evaluations and services. These include long waitlists for evaluations, lack of specialized expertise in community settings and provider hesitancy to refer young infants for further evaluation. Families are often told to ‘wait and see’. To help increase access to early evaluations, we have developed a telehealth-based early developmental evaluation for infants, the TEDI. Using video telecommunication technologies such as Zoom, families are able to connect with experts and access autism evaluations from their own homes. Importantly, developing a telehealth-based assessment for autism will increase access for families in rural and low-resourced areas.
In one of our research studies, we are examining whether this telehealth-based approach is feasible for clinicians and researchers in terms of our ability to capture key infant behaviors. We are also gathering feedback from families about whether the TEDI meets their needs, as well as any ideas for improvement. Preliminary findings suggest that examiner-scored behavioral ratings from video sessions were reliable and that infants’ behavior was stable across multiple observation sessions. Sessions were completed with minimal technological problems, and families rated the TEDI as highly acceptable in terms of the technology used, assessment components included, and the convenience of telehealth-delivered sessions. We are now examining these same features in a larger sample of infants, recruited nationwide, whose parents have concerns about their social communication development. Recruitment for this larger study is ongoing through September 2021.
In a second recently awarded grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, we will be examining another aspect of the TEDI. We need to see how the TEDI compares to existing standardized and validated measures collected in a traditional laboratory setting. This next step is crucial for demonstrating that this telehealth-based approach can be reliably used to evaluate infants’ behavior. We will be asking families who are receiving existing gold-standard in-person assessments through another project at the MIND Institute, the LAAMB study, to participate in an additional telehealth session using the TEDI from home. This will allow us to compare infants’ behavior across the two study settings. We’ll do this at three ages: 6 or 9 months, 12 months, and 18 months. Other MIND Institute faculty involved in this project include Meghan Miller, who directs the LAAMB study, and Gregory Young and Sarah Dufek. We are so grateful to all the families who have participated so far!
-
By Amanda Dimachkie Nunnally
Autism characteristics in individuals with Down syndrome
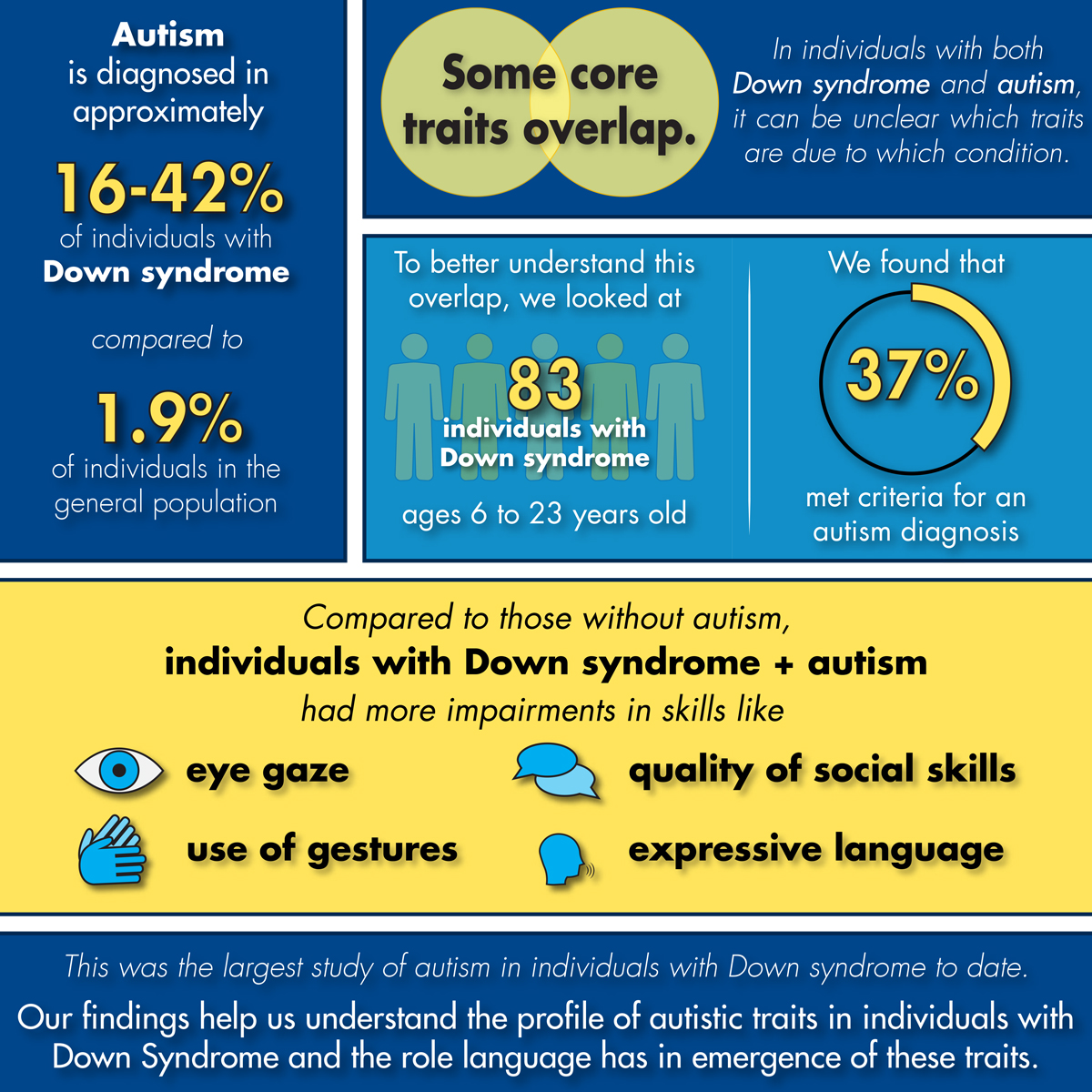
Individuals with Down syndrome are diagnosed with autism at a much higher rate than the general population, with prevalence estimates ranging from 16-42% in Down syndrome compared to 1.9% in the general population. There is still much to be understood about the presentation of autism symptoms in individuals with Down syndrome, as some of the core characteristics of autism may overlap with intellectual disability. Individuals with Down syndrome and co-occurring autism tend to have more severe rigid and repetitive behaviors and greater challenges with social communication than do individuals with Down syndrome alone. However, the degree to which symptoms can be attributed to each condition remains understudied.
In a recent study published in Brain Science, we explored the presence and presentation of autism characteristics in 83 individuals with Down syndrome, aged between 6 and 23 years. We found that 37% of our sample met criteria for a diagnosis of autism using the Autism Diagnostic Observation Schedule 2 (ADOS-2), consistent with what prior studies have reported. Next, we explored which skills on the ADOS-2 differentiated individuals in our sample who met autism classification from those who did not. Skills such as eye gaze, use of gestures, quality of social overtures and rapport were more impaired in individuals with Down syndrome who met autism classification compared to those who did not. This finding is consistent with the notion that while individuals with Down syndrome may experience difficulties in social communication, individuals with both Down syndrome and autism experience more substantial difficulties in this area. Lastly, upon examining whether the two groups differed in language and cognitive abilities, we discovered that individuals with Down syndrome who met classification for autism demonstrated more limited language skills, particularly expressive grammar, than those with Down syndrome alone.
Altogether, our findings confirm earlier studies reporting higher rates of co-occurring autism in individuals with Down syndrome. They also confirm more severe impairments on specific social measures and language skills in individuals with Down syndrome who met classification for autism compared to those who did not. Additionally, for individuals in our sample with more limited language skills, the presence of restricted and repetitive behaviors no longer differentiated individuals with Down syndrome who classified for autism from those who did not. This suggests shared characteristics between autism and Down syndrome. The findings presented in this study build upon earlier studies by examining the presentation of autism characteristics in individuals with Down syndrome using the largest sample to date, describing for the first time the profile of symptoms in these individuals, and establishing a role for expressive language in the emergence of autism symptoms. Our findings demonstrate the need for more research exploring the complexities of diagnosing autism in individuals with Down syndrome, particularly the relationship between language and the presentation of autism characteristics.
-
By David E. Olson
Engineering non-hallucinogenic versions of psychedelics
Changes in neuronal structure are at the heart of neuropsychiatric conditions such as depression, substance use disorder, and post-traumatic stress disorder (PTSD), as well as developmental conditions such as schizophrenia and autism. The development of safe medications that could reverse or repair these neuronal alterations is an active area of research. In 2018, David E. Olson and his lab discovered that psychedelics, like LSD and MDMA, promote neuroplasticity and facilitate the growth of cortical neurons—a finding that could explain why psychedelics produce long-lasting therapeutic effects after a single dose. Though psychedelics are demonstrating promising results for treating depression, PTSD, and social anxiety in adults with autism under carefully controlled and monitored conditions, the wider use of these treatments is limited by their hallucinogenic effects, potential for heart damage, and/or psychostimulant properties.
To solve this issue, the Olson Lab has engineered several non-hallucinogenic versions of psychedelics that produce therapeutic effects in preclinical models with improved safety profiles. Their non-hallucinogenic version of the drug ibogaine, known as tabernanthalog (TBG) was described in a paper published in Nature last December. It produces therapeutic effects in models of depression, opioid use disorder, and alcohol use disorder, though the compound has not yet been tested in humans.
More recently, the Olson Lab partnered with Lin Tian’s group to develop psychLight—the first ever optical test for determining the hallucinogenic potential of specific compounds . In a paper published in Cell, the team used psychLight to identify AAZ-A-154 (AAZ), a non-hallucinogenic MDMA-like compound with long-lasting antidepressant effects but no psychostimulant properties. Olson and his team are currently testing the effects of AAZ in experiments relevant to treating PTSD and social anxiety in adults with autism. Olson Lab research also led to the founding of Delix Therapeutics—a biotech company focused on using non-hallucinogenic analogs of psychedelics to treat a variety of neuropsychiatric and neurodegenerative conditions.
-
By Devon Gangi
Decline in looking at faces may signal onset of autism in infants
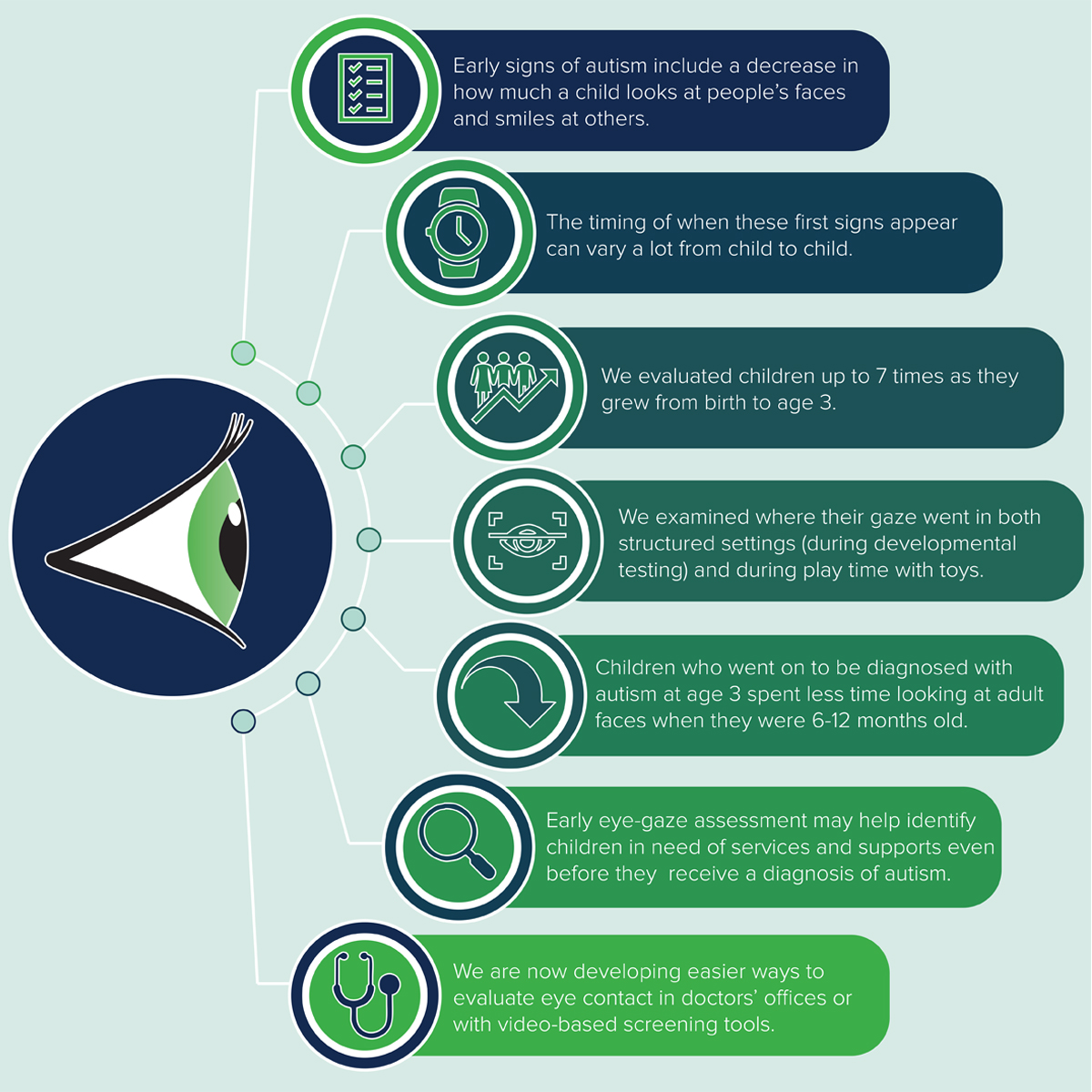
The first signs of autism usually appear in the first two years of life, but exactly when they occur can vary quite a bit from child to child. Our lab has followed infants from shortly after birth until age 3, through the window when autism characteristics develop. We see the children multiple times so that we can directly measure the timing of onset. Past studies that have similarly followed babies over the first few years of life have found that children who developed autism showed declines in social-communication behavior, such as looking at the faces of other people or smiling at others, between 6 and 36 months.
In 2010, the Ozonoff lab reported that infants who were diagnosed with autism at age 3 showed a decline in how often they directed their gaze toward an adult who was interacting with them as early as 12 months of age. However, that study was conducted in a relatively small group of children, so it was important to replicate these findings to make sure that they are accurate. In a new paper, we followed two independent groups of infants from 6 to 36 months (155 children in one group and 126 in the other) and examined their gaze behavior in different settings and contexts. This included interacting with an adult, either during developmental testing or during play with toys. We found the same results as the original study. Across both groups of children—and both settings—most children who were diagnosed with autism at 36 months showed sharp decreases in how often they looked at the faces of the adult they were interacting with relative to children without autism, including those with other developmental conditions, such as language delays. Children with autism showed lower levels of gaze to the adult’s face by 6-12 months of age than children who did not develop autism.
These findings add to our understanding of typical and atypical trajectories of social-communicative behaviors and the early development of autism characteristics, suggesting that declining developmental trajectories in some social-communicative behaviors across infancy may occur in most children diagnosed with autism by age 3. Key social behaviors, like looking at or smiling toward other people, could be tracked at every well-child visit and charted, much like doctors do for height and weight, to be sure that a child’s social development is on track and does not show declines. Such methods might allow children to be identified during the decline of skills and participate in helpful early intervention, even before meeting criteria for a full diagnosis of autism. This study used a time-intensive, laboratory-based method to examine social-communicative behaviors, and our lab is currently working on methods of evaluating these behaviors that could be applied outside the laboratory, such as in a doctor’s office, through a video-based screening tool.
-
By Olivia Taylor
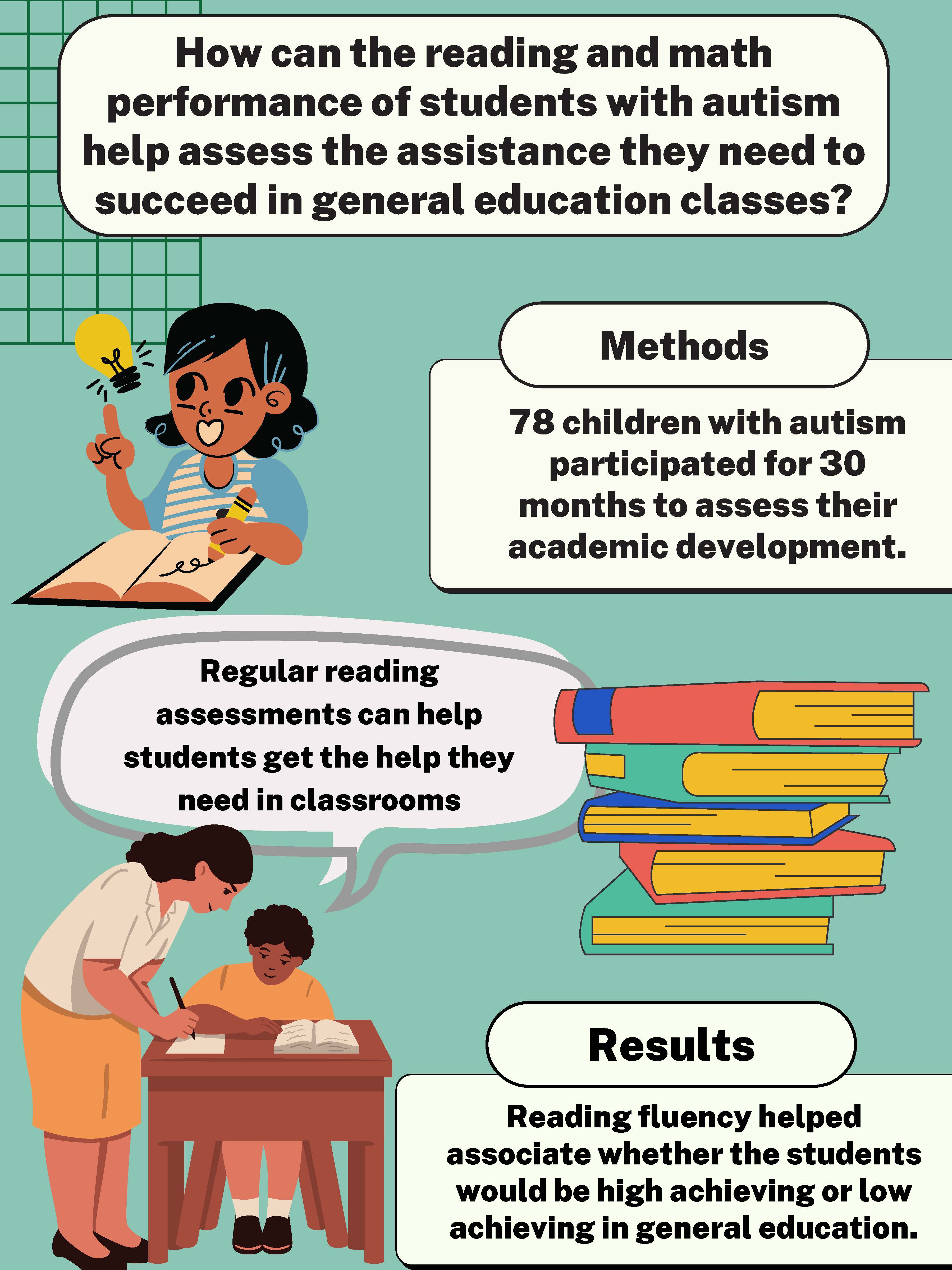
Math and reading achievement in children and adolescents with autism
There is limited research about the best ways to support students with autism in general education settings, particularly those that may have complications with their mathematics or reading skills. This study’s goal was to research further how performance on various cognitive and academic variables may be associated with achievement levels in general education spaces. These associations can be used to expand on the knowledge on accommodating diverse learners' needs.
A total of 78 children with autism aged 6-18 participated in this study for 30 months to assess their academic, social, and cognitive development; some students may have also had other clinical diagnoses such as attention-deficit/hyperactive disorder (ADHD) and anxiety. The students went through various reading fluency, reading comprehension, problem-solving, and other cognitive tests to assess potential performance levels in general education.
After assessing the data associated with the results of the academic and cognitive assessments, the students were classified into two achievement groups: high achieving and low achieving in respect to how they may perform in a general education classroom. Evidence showed that performance on reading fluency tests was the only factor that accurately associated students with their appropriate achievement groups.
As autistic students continue to integrate into general education classrooms, it is important that educators are able to assess how to best support them as they face challenges. As educators move forward, performance levels on reading fluency tests can be an important assessment tool in determining the proper support a student with autism may need in a general education classroom. Overall, the data revealed important information to lead educators on the right path in providing adequate support to students with autism. In the future, a more comprehensive study of a diverse range of students would better comprehend the needed support of a wider variety of students with autism in regards to race, socioeconomic status, and location.
-
By Rida Baig
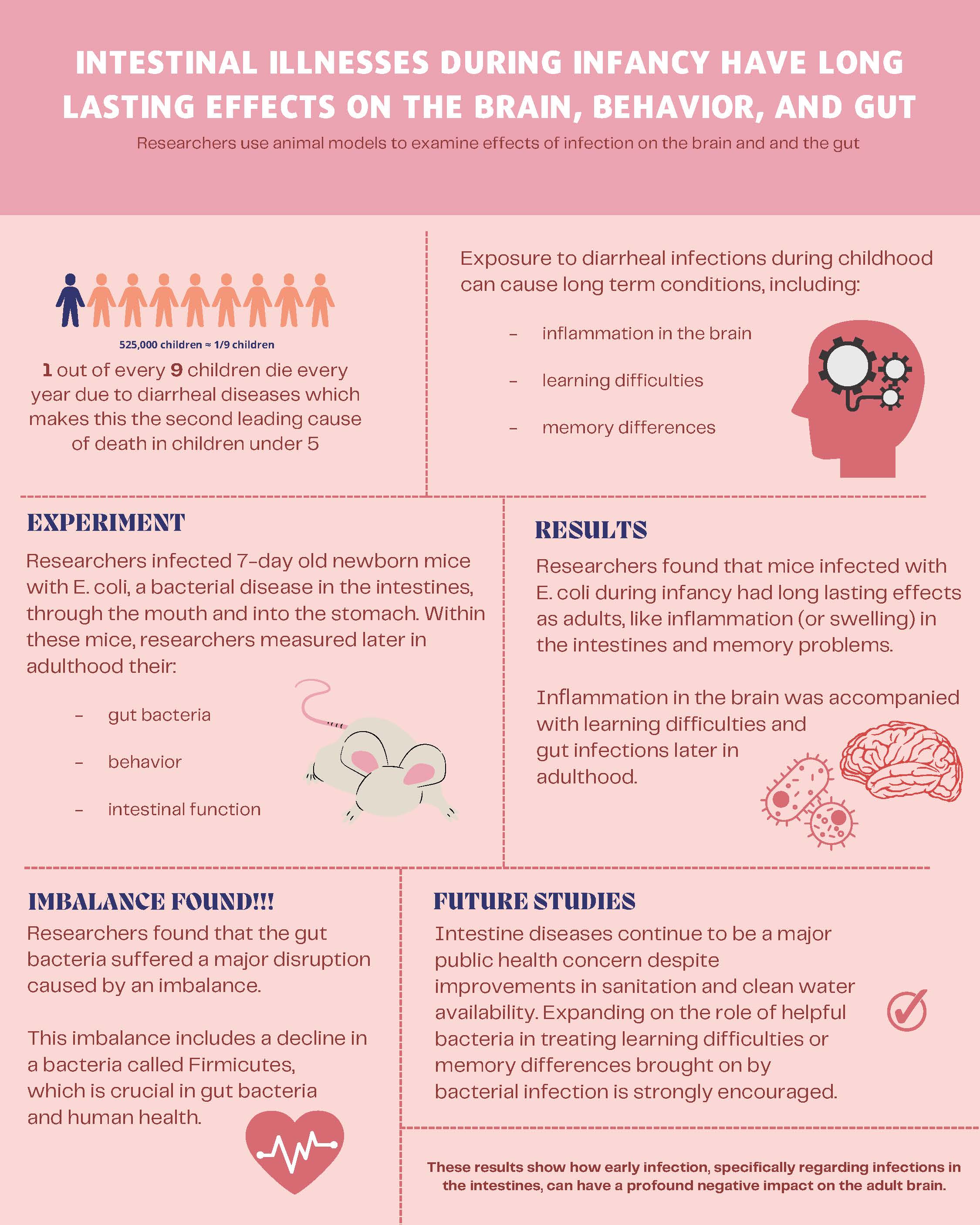
Intestinal illnesses during infancy have long lasting effects on the brain
The second leading cause for mortality amongst children under the age of five is diarrheal diseases. Exposure to diarrheal infections during childhood can cause chronic conditions, including cognitive abnormalities later in adolescence and adulthood.
To study this, researchers contaminated 7-day-old newborn mice with E. coli, a bacterial disease in the intestines, through the mouth and into the stomach. Researchers measured gut bacteria, behavior, and intestinal function within these mice later in adulthood. They found that the E. coli infected mice later displayed intestinal inflammation and decreased memory recall in adulthood. Enhanced regeneration and inflammation in the brain were accompanied with cognitive deficits.
They additionally found that the gut bacteria suffered a major disruption caused by an imbalance. This imbalance includes a decline in a bacteria called Firmicutes, which is crucial in gut bacteria and human health. These results show how early infection, specifically regarding infections in the intestines, can have a profound negative impact on the adult brain. Intestinal diseases continue to be a major public health concern despite improvements in sanitation and clean water availability. Expanding on the role of helpful bacteria in treating learning difficulties or memory differences brought on by bacterial infection is strongly encouraged.
-
By Matthew Bruce
The role of maternal autoantibody exposure in brain development in autism
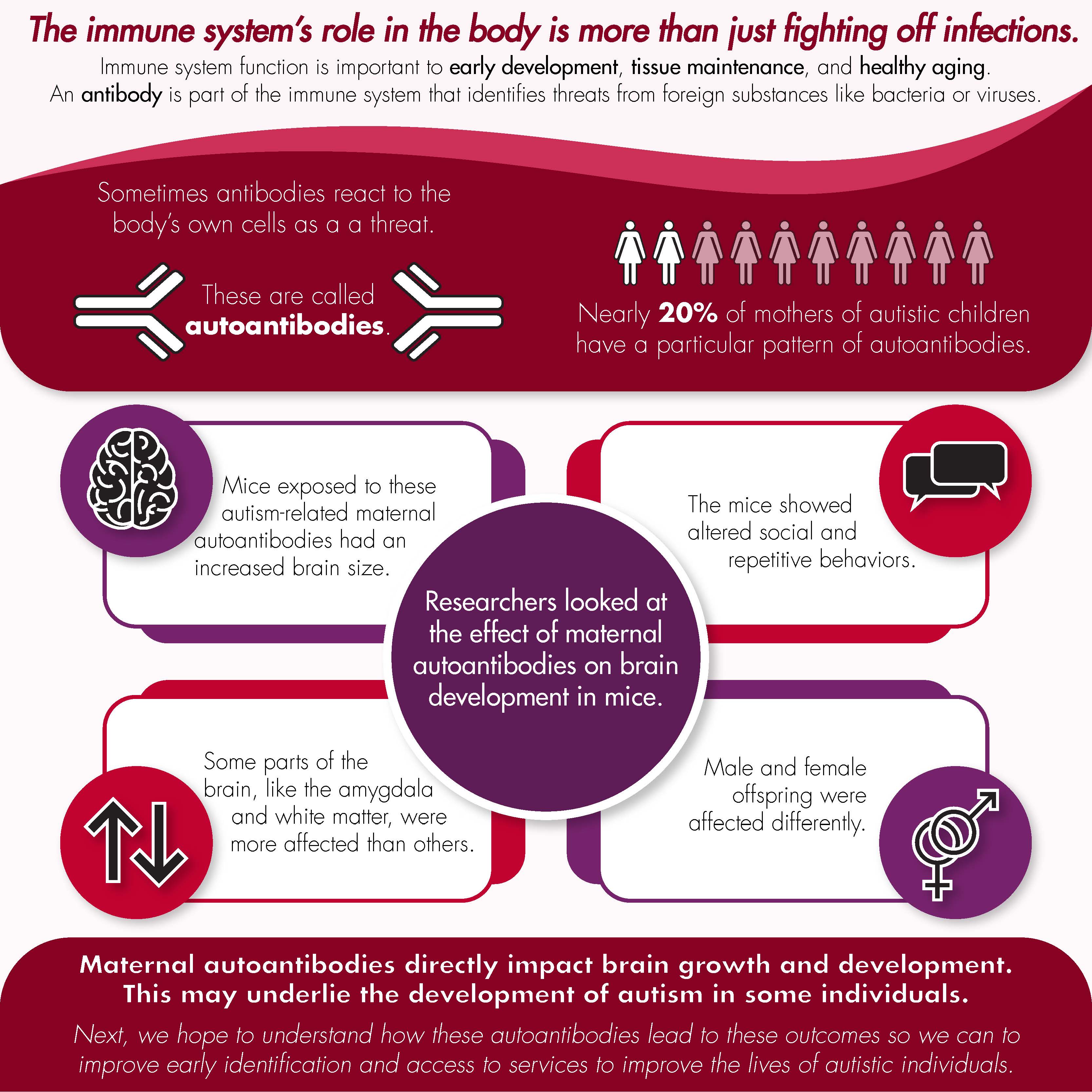
The immune system has a distinct role in many bodily functions across the lifespan, extending well beyond its capacity to fend off infections. Indeed, substantial research suggests that immune cells and their molecules are important orchestrators in early development, tissue maintenance, and healthy aging. Unsurprisingly, given this broad influence, dysregulation of the immune system has been linked to a multitude of conditions, including neurodevelopmental disabilities such as autism.
Research in Judy Van de Water’s lab has focused on understanding the role of the immune system in autism to provide insight about the biological basis of this neurodevelopmental condition. One major finding from this work has been that nearly 20% of mothers with children on the spectrum also present with specific patterns of circulating antibodies to self-proteins, known as autoantibodies.
A recent collaborative effort by MIND Institute Intellectual and Developmental Disabilities Research Center (IDDRC) investigators Judy Van de Water, Jill Silverman, and Jacqueline Crawley, as well as researchers from Canada and the United Kingdom, used an animal model to evaluate the effects of these maternal autoantibodies on offspring brain development. This study, published in July 2021 in the journal Molecular Psychiatry, found that mice exposed to autism-specific maternal autoantibodies during gestation displayed increased brain size, similar to that seen in autistic individuals born to mothers with these autoantibodies. It was also apparent that certain regions in the brain, such as the amygdala and white matter areas, were more affected by maternal autoantibody exposure than others. Finally, the differences in brain volume observed in mice also related to altered social and repetitive behaviors and affected male and female offspring differently.
These results suggest that maternal autoantibodies have a direct impact on the way that the brain grows and develops, and that these changes may underlie the development of autism in a subset of individuals. Current efforts are focused on understanding how these autoantibodies lead to altered outcomes using a combination of approaches. These include looking at the molecular and cellular level using mRNA sequencing and cell culture techniques. The overarching goal of our research program is to enhance the ability for early intervention and implementation of services to improve the lives of affected individuals.