OMOP
What is the OMOP Common Data Model (CDM)?
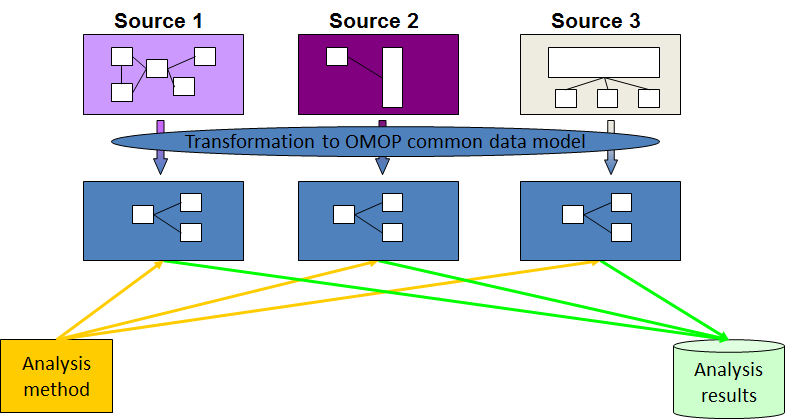
The OMOP Common Data Model allows for the systematic analysis of disparate observational databases. The concept behind this approach is to transform data contained within those databases into a common format (data model) as well as a common representation (terminologies, vocabularies, coding schemes), and then perform systematic analyses using a library of standard analytic routines that have been written based on the common format.
Why do we need a CDM?
Observational databases differ in both purpose and design. Electronic Medical Records (EMR) are aimed at supporting clinical practice at the point of care, while administrative claims data are built for the insurance reimbursement processes. Each has been collected for a different purpose, resulting in different logical organizations and physical formats, and the terminologies used to describe the medicinal products and clinical conditions vary from source to source.
The CDM can accommodate both administrative claims and EHR, allowing users to generate evidence from a wide variety of sources. It would also support collaborative research across data sources both within and outside the United States, in addition to being manageable for data owners and useful for data users.
Why use the OMOP CDM?
The Observational Medical Outcomes Partnership (OMOP) CDM, now in its version 6.0, offers a solution unlike any other. OMOP found that disparate coding systems can be harmonized—with minimal information loss—to a standardized vocabulary.
Once a database has been converted to the OMOP CDM, evidence can be generated using standardized analytics tools. We at OHDSI are currently developing Open Source tools for data quality and characterization, medical product safety surveillance, comparative effectiveness, quality of care, and patient-level predictive modeling, but there are also other sources of such tools, some of them commercial.
Read more about the OMOP CDM in the Book of OHDSI.
For more information about the CDM please read the documentation, download the DDL for various database dialects and learn about the Standardized Vocabularies. If you have questions post them at the OHDSI Forum.
A 1k sample of CMS SynPUF data in CDM Version 5 is available to download on LTS Computing LLC’s download site.
OMOP access request- OMOP Access Request
OMOP support, consultation and enhancements-OMOP Consultation
Enhancement and support for existing extracts/ETL processes, new ETL development, new data asset development-Data Asset Development and Enhancement
OMOP Quick Links:
Read More About the OMOP CDM in the Book of OHDSI
A 1k sample of CMS SynPUF data
UC Davis campus CAS/Kerberos account required.
UC Davis Health AD/Citrix account required.
