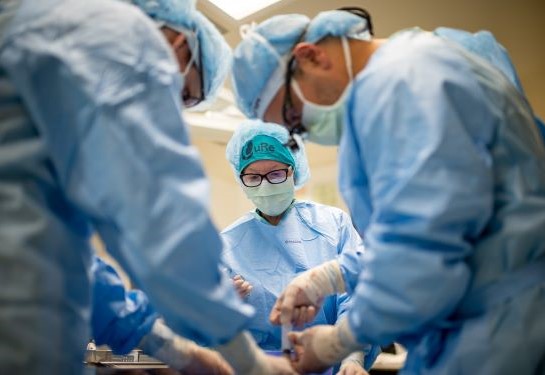
Diana Farmer wins top national award for innovative fetal spina bifida stem cell research
The fetal surgeon’s message to researchers and the latest on her groundbreaking CuRe clinical trial
This week, the Association for Clinical and Translational Science awarded UC Davis Distinguished Professor Diana Farmer the prestigious Edward H. Ahrens, Jr. Award for Outstanding Achievement in Patient-Oriented Research. This award recognizes her first-of-its-kind work which combines fetal surgery with stem cells to treat spina bifida. It also honors her excellence in moving basic research from laboratory to patients.
Farmer is an internationally renowned fetal and neonatal surgeon. She is the Pearl Stamps Stewart Endowed Chair and the chairperson of the UC Davis Department of Surgery. She is also the chief of pediatric surgery at Shriners Children —Northern California and founder and co-director of the Center for Surgical Bioengineering.
In this Q&A, Farmer shares insights from her landmark CuRe (Cellular Therapy for in Utero Repair) trial for spina bifida, her journey as a physician-scientist and the importance of believing in transformational research.
What does this translational award mean to you?
I have been in this clinical translational space my whole life. As physicians, the reason most of us do science is to improve the lives of our patients. That's the definition of clinical translational science.
So, I was completely surprised when I learned I had won this award. I'm hugely honored that they have taken notice of our work on this rare disease (spina bifida) and this little, small corner of the disease universe. Really thrilled!
What does it take to become a distinguished researcher making medical breakthroughs?
I can sum it up in two words: passion and persistence. You can't get discouraged by the experiments that don't work, the political roadblocks in your way, or funding obstacles. If you believe, you just have to push through all of it.
At every point in my career, even when I've been a little discouraged or disillusioned, I have found either a new collaborator, a new job or a new something that has restored my faith in science and kept me going through the hard times. Because, like all of life, there are ups and downs. Be creative. Find new solutions. Just don't give up. Believe in your vision.
You can't get discouraged by the experiments that don't work, the political roadblocks in your way, or funding obstacles. If you believe, you just have to push through all of it.” —Distinguished Professor Diana Farmer
Clinical trials require substantial research funding. Can you tell us about the support that the CuRe trial received and the importance of investing in breakthrough research and innovations?
The National Institutes of Health (NIH) funded my initial effort in spinal bifida which became the landmark MOMS (Management of Myelomeningocele Study) trial. MOMS proved that fetal surgery improved outcomes for children with spina bifida compared to surgery after birth. This work led to the current CuRe trial for spina bifida.
But when I applied for NIH funding to add stem cells to fetal surgery, it was turned down. So, I pivoted to the California Institute for Regenerative Medicine (CIRM) and then to Shriners Children’s to help fund this important work for these children.
How does your work on the CuRe trial feed into the bigger picture of discovery, innovation and finding cures at the prenatal stage of development?
As a prenatal and fetal surgeon, I would say that the most interesting aspect of medical discovery is the future of cell and gene therapy before birth. This is where the future lies.
Through the CuRe trial, we brought more attention to the benefits of trying to cure disease before birth, whether through gene editing or cellular therapies. We can impact a patient’s entire life, potentially eliminating diseases or influencing the adult manifestation of diseases that arise before birth.
Where are we now in the CuRe trial?
We are almost at the halfway point. We've passed the early safety component, and the FDA has allowed us to enroll patients a little faster. So, in a few years, we should have some answers.
Learn more about the CuRe trial.
Related resources: