New technology could increase donor kidneys for transplants
UC Davis Health researchers study novel technology to use kidneys previously considered unsuitable for transplant
Most people in need of a kidney wait three to five years on a national transplant list in the United States according to the National Kidney Foundation. The most current list has more than 103,000 people on it.
Over the years, transplant health care professionals have considered different options to increase the number of available kidneys. This includes using “suboptimal kidneys,” such as kidneys from donors who died after their circulatory and respiratory functions ceased, donors over 65, and those with diabetes and hypertension.
However, other than the general Kidney Donor Profile Index (KDPI) score, there are few reliable ways to see if a particular kidney is functional and suitable for a transplant.
But now, researchers at UC Davis Health are looking into an emerging method of easing the transplant waiting list: They are studying whether a procedure that extends the life of a donated kidney, after it’s been removed from the body, will expand the donor pool.
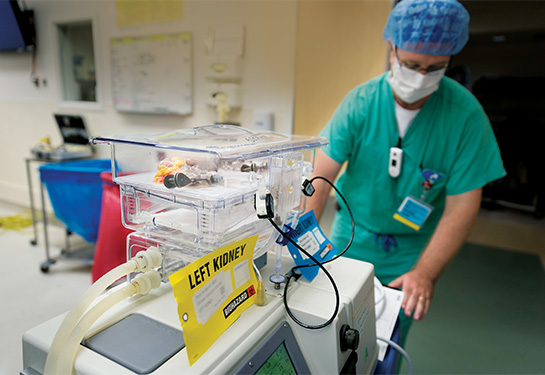
The procedure is called ex vivo normothermic perfusion.
What is ex vivo normothermic perfusion?
Traditionally, donated organs are placed on ice, then quickly transplanted into a recipient. With ex vivo normothermic perfusion, a machine keeps organs warm by continuously pumping blood through them.
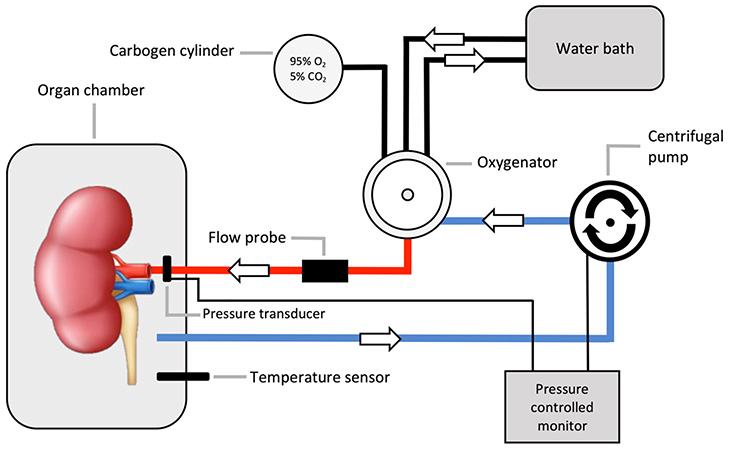
The process involves the donated kidney being placed inside a sterile steel container attached to a ventilator, pump and filters. Within the container, the kidney is maintained at normal body temperature and perfused with reagents that include insulin, creatinine, vitamins, glucose and a cocktail of essential and non-essential amino acids that are used as the energy source of the kidney.
Oxygenated packed red blood cells are warmed by a water bath that flows through the kidney via the renal artery cannula. The red blood cells exit from the kidney into a centrifuge where they are oxygenated, warmed, and recirculated back to the kidney.
“This procedure allows the kidney to spend much less time on ice without blood flow, which is better for the organ,” explained Armin Ahmadi, postdoctoral scholar of nephrology at UC Davis Health and lead-author of a new study on ex vivo normothermic perfusion. “Additionally, during the procedure we are able to better assess the quality and function of the kidney before it is transplanted — improving the likelihood of a successful transplant.”
During the procedure we are able to better assess the quality and function of the kidney before it is transplanted – improving the likelihood of a successful transplant.” —Armin Ahmadi
The UC Davis researchers set out to assess the promising ex vivo normothermic perfusion method to potentially expand the donor pool. Their goal was to identify the ideal amount of time for the process and to identify metabolic markers for the most suitable kidneys for transplantation.
Their findings were published in the premier nephrology journal Kidney International. The research team concluded that perfusing kidneys for six hours proved to be the ideal timeframe to accurately assess their suitability. Additionally, their exploration into metabolic profiles unveiled a crucial factor in a well-functioning donor kidney — metabolic flexibility, particularly in the handling of branched chain amino acids.
By refining perfusion processes and focusing on these metabolic markers, our research represents a significant stride toward mitigating the shortage of donor kidneys in the field of transplantation.” —Baback Roshanravan
What the study showed
The study analyzed eight human kidneys from eight deceased donors deemed unsuitable for transplantation. Researchers put each kidney through a 12-hour ex vivo normothermic perfusion, during which they collected and assessed tissue of the kidneys for metabolic function every hour. The kidneys were then grouped into “good” and “poor” performers based on blood flow and urine output. Researchers found urine output and creatinine clearance progressively increased and peaked at six hours post-perfusion and labeled as good performers.
Additionally, looking into kidney tissue metabolic profiles showed a crucial factor in a well-functioning donor kidney is metabolic flexibility, particularly in the handling of branched chain amino acids.
“This newfound insight provides valuable information on the factors to determine kidney suitability for transplantation, Ahmadi said. “In addition to extending the operating window of time by six hours, we also have information on how we can improve the metabolic functions of the kidney during that time.”
Although the results are promising, the researchers note that the study size was small, and more research will be needed to optimize the perfusion method.
Additional authors on the study include Naeem Goussous, Brian C. Howard, Jennifer E. Loza, Ivonne Palma, Richard V. Perez, Junichiro Sageshima and Jacquelyn Yu from the Division of Transplant Surgery at UC Davis Health.